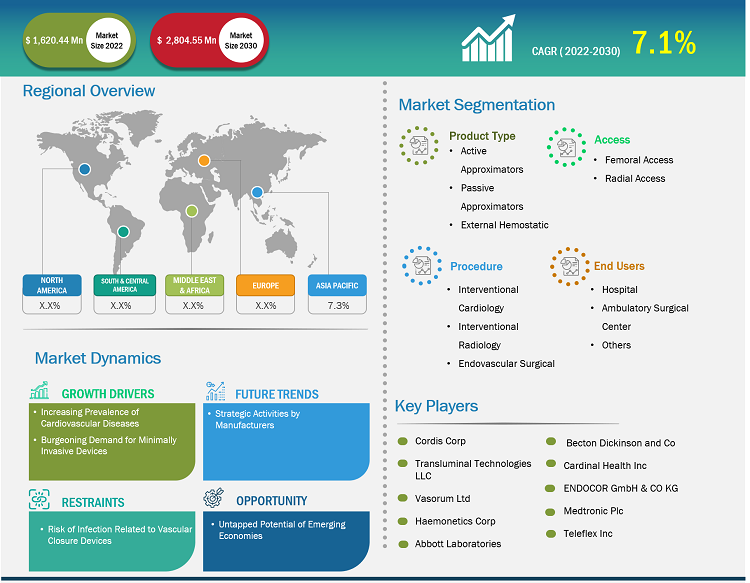
The vascular closure device market is expected to reach US$ 2,804.55 million by 2030 from US$ 1,620.44 million in 2022. The market is estimated to grow with a CAGR of 7.1% from 2022 to 2030.
Market Insights and Analyst View:
A vascular closure device is a piece of collagen (a fibrous protein found in skin, connective tissue, and bone), a metallic clip, or a suture designed to close the small puncture produced in an artery following an angiography. Key factors driving the vascular closure device market growth include the increasing prevalence of cardiovascular diseases and burgeoning demand for minimally invasive devices. However, the risk of infection related to vascular closure devices hinders the market growth.
Growth Drivers and Restraints:
Cardiovascular diseases such as atherosclerosis, angina pectoris, and acute myocardial infarction are the major causes of mortality in the world. As per data provided by the World Health Organization (WHO), cardiovascular diseases cause nearly 17.9 million deaths every year, which makes it a leading cause of mortality among all noncommunicable diseases, followed by cancer, chronic respiratory diseases, and diabetes (including deaths associated with renal disorders). According to the World Heart Federation, high cholesterol causes 4.4 million deaths yearly, and ∼24% of cardiovascular-related deaths are associated with high low-density lipoprotein (LDL) levels. According to a study published in January 2023 by NCBI, in the US, the number of people aged 50 years and older is likely to reach 221.13 million by 2050 from 137.25 million in 2020. Among people from this age group, the number of people suffering from at least one chronic disease is projected to rise by 99.5%, reaching 142.66 million by 2050 from 71.52 million in 2020. Moreover, ~14 million people of age 50 and above are likely to have comorbidities in 2050.
Vascular closure devices (VCDs) offer a new way to enhance patients’ comfort and ambulation after invasive cardiovascular treatments via femoral arterial access. These devices are used in different therapeutic approaches to provide easy, quick, and reliable hemostasis. In the US, femoral arterial access is the most often used vascular access technique for percutaneous coronary intervention (PCI) and coronary angiography. Nevertheless, there has been an increase in traction toward cardiac catheterization and percutaneous coronary intervention (PCI) via radial access in recent years. Several femoral artery closure devices have been developed to shorten vascular closure times, with variable rates of vascular complications observed in clinical trials. VCDs have emerged as an effective alternative to traditional mechanical compression procedures performed after cardiac catheterization. VCDs reduce the time of hospital stays, speed up patient mobilization, and shorten the time needed to achieve hemostasis. Thus, the vascular closure device market is growing notably with the increasing prevalence of cardiovascular diseases.
On the other hand, vascular closure devices are more prone to causing infections as compared to manual compression. Groin hematomas are among the most common problems that can occur just after sheath removal upon failure to control the femoral artery while using femoral vascular closure devices. In cases of vascular closure device failure, manual compression must be applied to accomplish hemostasis. Groin hematomas are the most common complication with femoral access, and patients who develop a hematoma should be seriously refrained from normal physical activities. Thus, groin hematomas may lead to incremental healthcare costs due to complications and extended length of hospital stays. Other notable complications of closure devices include limb ischemia and ischemia distal to the arteriotomy site. Distal limb ischemia commonly occurs through distal embolization or thrombosis, and it can be caused due to the placement of a closure device, which is contraindicated in heavily calcified areas of arteries. Compared to manual compression, limb ischemia infection is more common in vascular closure devices. Thus, the risk of infection hampers the growth of the vascular closure device market to a certain extent.
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
Vascular Closure Device Market: Strategic Insights
Market Size Value in US$ 1,620.44 million in 2022 Market Size Value by US$ 2,804.55 million in 2030 Growth rate CAGR of 7.1% from 2022 to 2030 Forecast Period 2022-2030 Base Year 2022

Akshay
Have a question?
Akshay will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
Vascular Closure Device Market: Strategic Insights

| Market Size Value in | US$ 1,620.44 million in 2022 |
| Market Size Value by | US$ 2,804.55 million in 2030 |
| Growth rate | CAGR of 7.1% from 2022 to 2030 |
| Forecast Period | 2022-2030 |
| Base Year | 2022 |

Akshay
Have a question?
Akshay will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Report Segmentation and Scope:
The global vascular closure device market is segmented on the basis of product type, access, procedure, and end user. Based on product type, the market is divided into active approximators, passive approximators, and external hemostatic devices. The vascular closure device market, by access, is bifurcated into femoral access and radial access. Based on procedure, the market is segmented into interventional radiology, interventional cardiology, and endovascular surgical. In terms of end user, the vascular closure device market is segmented into hospitals, ambulatory surgical centers, and others. The vascular closure device market, based on geography, is segmented into North America (US, Canada, and Mexico), Europe (Germany, France, Italy, UK, Russia, and Rest of Europe), Asia Pacific (Australia, China, Japan, India, South Korea, and Rest of Asia Pacific), Middle East & Africa (South Africa, Saudi Arabia, UAE, and Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and Rest of South & Central America).
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
Segmental Analysis:
The vascular closure device market, by product type, is segmented into active approximators, passive approximators, and external hemostatic devices. The passive approximators segment held the largest share of the market in 2022, and it is anticipated to register the highest CAGR in the market during 2022-2030. Passive approximators deploy a plug, sealant, or gel at the arteriotomy site instead of closing it via an active mechanism. Unlike active approximators, passive approximators do not require any additional closure mechanisms, such as sutures or clips, once deployed. Passive approximators typically consist of biodegradable or resorbable components that facilitate the natural clotting process and promote the formation of a stable blood clot at a puncture site.
Vascular Closure Device Market, by Product Type – 2022 and 2030

- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
Based on access, the vascular closure device market has been segmented into femoral access and radial access. The femoral access segment held a larger share of the market in 2022. The radial access segment is expected to register a higher CAGR in the market during 2022–2030. Femoral access involves accessing blood vessels through the femoral artery in the groin area, while radial access involves accessing blood vessels through a radial artery in the wrist. The radial approach has gained popularity in recent years due to its advantages such as reduced risk of complications, including bleeding and hematoma, compared to femoral access.
Based on procedure, the vascular closure device market is segmented into interventional radiology, interventional cardiology, and endovascular surgical. The interventional cardiology segment held the largest share of the market in 2022. However, the interventional radiology segment is expected to register the highest CAGR in the market during 2022–2030. Interventional radiology (IR) is a medical specialty that uses minimally invasive techniques guided by medical imaging to diagnose and treat a wide range of conditions.
Based on end user, the vascular closure device market is segmented into hospitals, ambulatory surgical centers, and others. The hospital segment held the largest share of the market in 2022. The ambulatory surgical centers segment is further expected to register the highest CAGR in the market during 2022–2030. Hospitals are the primary end users of vascular closure devices as they offer a wide range of medical services, including invasive procedures that require vascular access.
Regional Analysis:
Based on geography, the vascular closure device market is segmented into North America, Europe, Asia Pacific, South & Central America, and Middle East & Africa. In 2022, North America held the largest share of the global vascular closure device market. Asia Pacific is estimated to register the highest CAGR during 2022–2030.
The US is estimated to hold the largest share of the vascular closure device market during 2022–2030. The country has a well-developed healthcare sector with access to highly advanced equipment and instruments. It reports a high prevalence of cardiovascular diseases. According to the “Heart Disease and Stroke Statistics - 2023 Update” by the American Heart Association, in 2020, coronary heart disease (CHD) was a leading cause (41.2%) of deaths associated with CVDs in the US, followed by stroke (17.3%), other CVD (16.8%), high blood pressure (12.9%), heart failure (9.2%), and artery diseases (2.6%), respectively. As per the US Centers for Disease Control and Prevention (CDC), ~1 in 20 adults in the US, aged 20 and above, have coronary artery disease. The study also mentions coronary artery disease as the most common type of heart disease in the US. Thus, a high prevalence of CVDs and coronary artery disease would propel the adoption of vascular closure devices during surgical interventions in the US in the coming years.
The US is a hub for medical innovations and technological advancements in healthcare. Continuous advancements in technologies associated with minimally invasive procedures, improvements in closure device technologies, and the introduction of novel closure devices contribute to the growth of the vascular closure devices market in the US. For instance, in March 2023, Haemonetics Corporation (US) invested ~US$ 31.72 million (EUR 30 million) in Vivasure Medical Limited. In March 2023, Vivasure Medical announced FDA IDE approval to Initiate a US Pivotal Study evaluating the safety and effectiveness of the Vivasure PerQseal Closure Device System. In 2021, VASCADE MVP was the first and only FDA-approved vascular closure device indicated for use following atrial fibrillation (AF) ablation to allow same-day discharge.
Industry Developments and Future Opportunities:
Various initiatives by key players operating in the global vascular closure device market are listed below:
- In August 2023, Haemonetics Corp announced that its VASCADE MVP Venous Vascular Closure system was used for the first time in Germany in treatments. This marked the first use of VASCADE systems, which are used widely in hospitals in the US, in a European country.
- In June 2023, Becton Dickinson and Co. opened a new manufacturing facility in Tijuana. The new 15,775-square-meter facility produces devices and other products to improve medication safety within healthcare settings.
- In May 2023, Cardinal Health Canada planned to open a new distribution center in Greater Toronto, strategically expanding its distribution channel with the addition of 9 other locations to better meet the medical and surgical product demands of the Canadian healthcare system.
- In September 2022, Haemonetics Corp earned CE mark certification for its VASCADE vascular closure and VASCADE MVP venous vascular closure systems.
- In May 2022, Teleflex Inc, a global provider of medical technologies, received Health Canada approval for the MANTA Vascular Closure Device—the first commercially available biomechanical vascular closure device designed specifically for large bore femoral arterial access site closure. This approval marks an important milestone in the plan of Teleflex to expand the availability of the MANTA globally, followed by apt regulatory approvals, thereby providing healthcare systems in Canada and in the world access to its another uniquely designed device.
- In August 2020, Vasorum Ltd signed a distribution agreement with Veryan Medical. Under the agreement, Veryan Medical supported Vasorum Ltd to commercialize the Celt ACD in the US market. Moreover, the agreement allowed both companies to strengthen their presence in the US market.
Competitive Landscape and Key Companies:
Cordis Corp, Transluminal Technologies LLC, Vasorum Ltd, Haemonetics Corp, Abbott Laboratories, Becton Dickinson and Co, Cardinal Health Inc, ENDOCOR GmbH & CO KG, Medtronic Plc, and Teleflex Inc are among the prominent players operating in the vascular closure device market. These companies focus on new technologies, advancements in existing products, and geographic expansions to meet the growing consumer demand worldwide and increase their product range in specialty portfolios.

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product Type, Access, Procedure, and End User

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
Based on the procedure, the vascular closure device market is segmented into interventional radiology, interventional cardiology, and endovascular surgical. The interventional cardiology segment held the largest share of the market in 2022.
The vascular closure device market is expected to be valued at US$ 2,804.55 million in 2030.
Based on the end users, the vascular closure device market is segmented into hospitals, ambulatory surgical centers, and others. The hospital segment held the largest share of the market in 2022.
Based on access, the vascular closure device market has been segmented into femoral access and radial access. The femoral access segment held the largest share of the market in 2022. However, radial access is expected to register the highest CAGR in the market during 2022-2030.
The vascular closure device market, by product type, is segmented into active approximators, passive approximators, and external hemostatic devices. The passive approximators segment held the largest share of the market in 2022, and it is anticipated to register the highest CAGR in the market during 2022-2030.
A vascular closure device is a piece of collagen (a fibrous protein found in skin, connective tissue, and bone), a metallic clip, or a suture designed to close the small puncture produced in an artery following an angiography.
The vascular closure device market majorly consists of the players, including Cordis Corp, Transluminal Technologies LLC, Vasorum Ltd, Haemonetics Corp, Abbott Laboratories, Becton Dickinson and Co, Cardinal Health Inc, ENDOCOR GmbH & CO KG, Medtronic Plc, and Teleflex Inc.
Factors such as increasing prevalence of cardiovascular diseases and burgeoning demand for minimally invasive devices propel market growth.
The vascular closure device market was valued at US$ 1,620.44 million in 2022.
The List of Companies - Vascular Closure Device Market
- Cordis Corp
- Transluminal Technologies LLC
- Vasorum Ltd
- Haemonetics Corp
- Abbott Laboratories
- Becton Dickinson and Co
- Cardinal Health Inc
- ENDOCOR GmbH & CO KG
- Medtronic Plc
- Teleflex Inc
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
Trends and growth analysis reports related to Vascular Closure Device Market

Nov 2023
Colonoscopes Market
Forecast to 2028 - COVID-19 Impact and Global Analysis By Product Type (Fiber Optic Colonoscopy Devices, Video Colonoscopy Devices); Application (Colorectal Cancer, Lynch Syndrome, Ulcerative Colitis, Crohn's Disease, Polyp); End User (Hospitals, Ambulatory Surgery Center, Others), and Geography

Nov 2023
Noninvasive Fat Reduction Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Technology (Cryolipolysis, Laser Lipolysis, Ultrasound, and Others), End User (Hospitals, Dermatology Clinics & Cosmetic Clinics, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Nov 2023
Medical Ultrasound Flow Meter Market
Size and Forecast (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Implementation Type (Clamp-On, Inline, and Others), Technology (Doppler, Transit Time, and Hybrid), Application (Heart and Lung Machines, Extracorporeal Membrane Oxygenation, Perfusion, Organ Transportation Systems, and Others), End User (Hospitals and Clinics, Ambulatory Surgical Centers, Research Laboratories, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Nov 2023
Rapid Test Kits Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Rapid Antigen Testing, Rapid Antibody Testing, and Others), Product (Over-the-Counter Rapid Testing Kit and Professional Rapid Testing Kit), Technology (Lateral Flow Assay, Solid Phase, Agglutination, Immunospot Assay, and Cellular Component-Based), Application (Blood Glucose Testing, Infectious Disease Testing, Pregnancy and Fertility, Cardiometabolic Testing, and Others), End User (Hospital and Clinics, Home Care, Diagnostics Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Nov 2023
Osteoarthritis Therapy Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Therapy Type [Transcutaneous Electrical Nerve Stimulation (TENS), Occupational Therapy, Physical Therapy, Platelet-Rich Plasma Therapy and Stromal Vascular Fraction, Prolotherapy, and Others], Disease Indication (Knee Osteoarthritis, Spine Osteoarthritis, Foot and Ankle Osteoarthritis, Shoulder Osteoarthritis, Hand Osteoarthritis, and Others), End User (Hospitals & Clinics, Specialty Clinics, Ambulatory Surgical Centers, Homecare, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Nov 2023
Bariatric Surgeries Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Type [Adjustable Gastric Bands (AGB), Sleeve Gastrectomy, Gastric Bypass, Biliopancreatic Diversion with Duodenal Switch (BPD-DS), and Others], End User (Hospitals and Ambulatory Surgical Centers), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Nov 2023
Post-Acute Care Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Service Type (Skilled Nursing Facilities, Inpatient Rehabilitation Facilities, Long-Term Care Hospitals, Home Health Agency, and Others), Age (Elderly, Adult, and Others), Disease Conditions (Amputations, Wound Management, Brain Injury and Spinal Cord Injury, Neurological Disorders, and Others), and Geography

Nov 2023
Lung Cancer Therapy Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Therapy Type (Noninvasive and Minimally Invasive), Indication (Non-Small Cell Lung Cancer and Small Cell Lung Cancer), End User (Hospitals, Oncology Clinics, Research Centers, and Others), and Geography (North America, Europe, Asia Pacific, South & Central America, and Middle East & Africa)