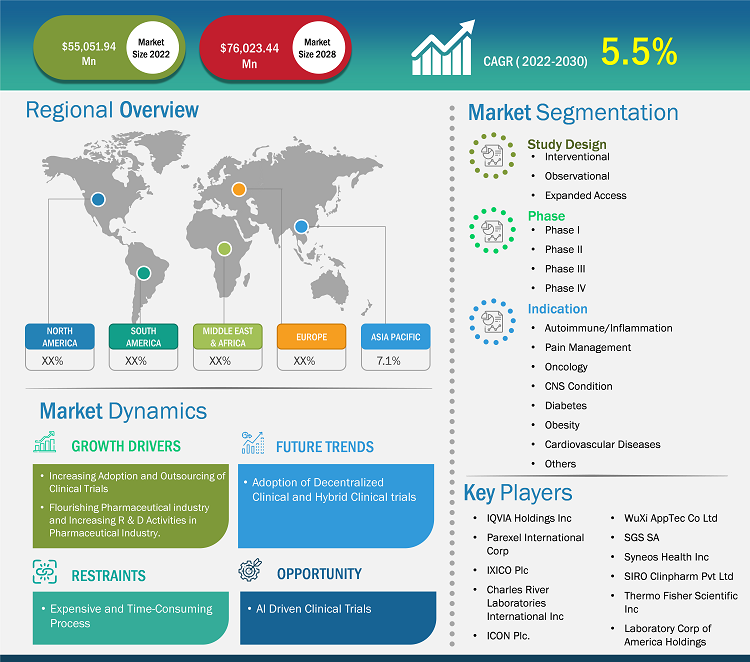
The clinical trials market size market was valued at US$ 55,051.94 million in 2022 and is projected to reach US$ 76,023.44 million by 2028; it is estimated to record a CAGR of 5.5% from 2023 to 2028.
Market Insights and Analyst View:
Clinical trials are research studies conducted to test a medical, surgical, or behavioral intervention in people. These clinical research trials are the primary ways of determining the safety and effectiveness of a new treatment or prevention (such as a new drug, diet, or medical device) in people. A clinical trial is often designed to learn if a new treatment is more effective or has less harmful side effects than existing treatments.
The clinical trial market is mainly driven by continuous efforts made by pharmaceutical and biotechnology, and medical device companies for product innovations. Other factors contributing to the market progress include the globalization of clinical trials, rapid advancements in associated technologies, and increased demand for CROs for conducting clinical trials. Furthermore, conferences such as “Global Clinical Trials Connect 2022” offer companies in this marketplace with a platform to get acquainted with advancements in clinical trials and clinical research. However, the rapidly increasing costs of conducting trials hinder the clinical trial market growth.
Growth Drivers and Challenges:
Increasing Adoption and Outsourcing of Clinical Trials
Clinical trials are performed to determine the safety, effectiveness, and efficacy of new treatments, prevention methods, and medical devices. The trials are mainly carried out during drug development. According to the data provided by the National Library of Medicine (NLM), ~52,000 new studies were registered with NLM (ClinicalTrials.gov) in 2020, which increased to ~58,000 in 2023. In January 2023, the NLM reported 38,837 active clinical trials in the US and 105,172 active trials worldwide. According to the European Medicine Agency, ~4,000 clinical trials are authorized annually in the European Union (EU), of which ~60% of clinical trials are associated with the pharmaceutical industry. An increasing number of clinical trials for developing different effective treatments due to the rising prevalence of chronic diseases globally is fueling the growth of the clinical trials market. Further, clinical trials are highly complex processes. Research-based organizations may fail to properly execute these processes or oversee the operations. To avoid errors due to improper execution, research-based organizations outsource clinical trials to clinical research organizations (CROs), which utilize their high-quality facilities and deep subject matter expertise to carry out clinical trials. CROs have begun acting as a backbone of the clinical trial industry through their efficient and cost-effective operations benefitting trial sponsors. According to a blog published on the Thermo Fisher Scientific website, in 2022, ~3 out of 4 clinical trials were carried out by CROs to reassure the clinical programs of drug developers, provide a wealth of expertise, drive time and cost efficiencies, and deliver customized, high-quality data. Thus, the development of cost-effective solutions and decreasing errors in CROs during the drug development process are among the factors driving the clinical trials market size.
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
Clinical Trials Market: Strategic Insights
Market Size Value in US$ 55,051.94 million in 2022 Market Size Value by US$ 76,023.44 million by 2028 Growth rate CAGR of 5.5% from 2023 to 2028 Forecast Period 2023-2028 Base Year 2023

Akshay
Have a question?
Akshay will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Customize Research To Suit Your Requirement
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
Clinical Trials Market: Strategic Insights

| Market Size Value in | US$ 55,051.94 million in 2022 |
| Market Size Value by | US$ 76,023.44 million by 2028 |
| Growth rate | CAGR of 5.5% from 2023 to 2028 |
| Forecast Period | 2023-2028 |
| Base Year | 2023 |

Akshay
Have a question?
Akshay will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
 Speak to Analyst
Speak to Analyst
Expensive and Time-Consuming Process
The rising number of therapeutic molecules in the last phases of clinical trials has cost money and valuable time invested in the research and development of new drugs. According to the article “Importance of ADME/Tox in Early Drug Discovery,” published in year 2022, considering the current drug discovery and development pipeline, out of 1,000 molecules, only 10 screened hits are projected to enter the preclinical testing stage, and only 9.6% will enter clinical trials. The drug approval process is projected to take 15 years on average, and phases II and III are the most expensive phases of clinical trials. The preapproval expenditures for a drug is as high as US$ 2.56 billion, which further increases to US$ 2.87 billion in the post-approved stage. According to the study titled “Why 90% of Clinical Drug Development Fails and How to Improve It?”, published in year 2022, 90% of clinical drug development fails despite the implementation of many strategies. After entering clinical investigations, nine out of ten drug candidates fail during phase I, II, and III clinical trials and the drug approval process. Drug candidates rejected in preclinical stages are not included in the 90% failure rate of the drugs in clinical stages, as they don’t even enter the phase I clinical trials. If preclinical drug candidates are considered, the drug discovery and development failure rate will rise above 90%. New drug development is resource and time intensive, whereas later clinical stages result in significant costs. The discovery and development of each new drugs approved for clinical use is a lengthy, expensive, and challenging process that takes over 10–15 years and costs, on average, over US$ 1–2 billion. Due to such high investment costs for developing drugs or medical devices, limited companies invest in clinical trial processes, which is hampering the growth of the clinical trials market.
Report Segmentation and Scope:
The “Global Clinical Trials Market” is segmented based on study design, phase, indication, and geography. Based on study design, the clinical trials market is segmented into interventional, observational, and expanded access. Based on phase, the clinical trials market is segmented into Phase I, Phase II, Phase III, and Phase IV. Based on End Use, the clinical trials market is segmented into autoimmune/inflammation, pain management, oncology, CNS condition, diabetes, obesity, cardiovascular and others.
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
Segmental Analysis:
Based on study design, the clinical trials market is into interventional, observational, and expanded access. The interventional segment held the largest share of the market in 2022, whereas the same segment is anticipated to register the highest CAGR of 5.8% in the market during the forecast period. An interventional study is a type of clinical research study wherein human participants receive one or more interventional drugs/treatments for a specific period to evaluate the effects of the drug/treatment on health. New potential drugs/medical devices/therapies must pass through phases of interventional testing to prove they are safe and effective before receiving approval from the Food and Drug Administration (FDA). Most of the interventional studies are placebo-controlled, where a group of participants receives a placebo, and the other group receives a drug under investigation. Researchers then compare the two groups to evaluate the drug's effectiveness under investigation.
Global Clinical Trials Market, by Study Design– 2022 and 2028

- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
Phase Insights


- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
Based on phase, the clinical trials market is segmented into Phase I, Phase II, Phase III, and Phase IV. The Phase III segment held the largest share of the market in 2022 and is estimated to register the highest CAGR in the market during the forecast period. Phase III clinical trial stage is carried out in large patient groups. The stage assists in determining the short-term and long-term efficacy of the active pharmaceutical ingredients. Thus, the assessment is done to know the total and associated therapeutic values of formulated drug. It is essential to maintain the efficacy, safety, and accuracy of the drug used in phase III clinical trials, which otherwise may adversely affect the patients under trials. Shertech Manufacturing offers phase III clinical trial services and comprehensive, definitive data related to efficacy and side effects to its customers. It also assists in complying with FDA standards that help introduce the drug into the market and complete the required licensing applications.
Indication Insights
The clinical trials market, by indication, has been segmented into autoimmune/inflammation, pain management, oncology, CNS condition, diabetes, obesity, cardiovascular disease, and others. The oncology segment held the largest clinical trial market share of the market in 2022 and is estimated to register the highest CAGR in the market during the forecast period. The increasing prevalence of cancer and the rising geriatric population contribute to the clinical trials market growth for the oncology segment. The American Cancer Society has estimated that in 2023, around 1,958,310 new cancer cases and 609,820 cancer deaths will occur in the US. Despite the significant progress in cancer diagnosis, prevention, and treatment procedures, there is a significant unmet medical need for treatments for primary cancers (e.g., prostate, lung, breast, and colon cancer). This is likely to increase cancer research and development activities. Pharmaceutical companies are focusing on developing new drugs to meet consumer needs. A clinical trial conducted in Tata Memorial Hospital, Mumbai, India, has found that an ultra-low dose of the immunotherapy drug nivolumab (Opdivo) was added to the standard treatment for head and neck cancer, which helped the patients to live longer.
Regional Analysis:
Based on geography, the clinical trials market is segmented into North America (US, Canada, and Mexico), Europe (UK, Germany, France, Italy, Spain, and Rest of Europe), Asia Pacific (China, Japan, India, Australia, South Korea, and Rest of Asia Pacific), the Middle East (UAE, Saudi Arabia, South Africa, and Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and Rest of South & Central America). North America accounted for the largest clinical trials market share during the forecast period. Asia Pacific is expected to register the fastest CAGR in the clinical trials market during the forecast period. China, Japan, and India are the prime contributors to the market in APAC. Thus, the clinical trials market in APAC is driven by growing investments from international players, burgeoning government support, and advancing healthcare infrastructure.
Industry Developments and Future Opportunities:
- In January 2023, Rznomics announced a partnership with Charles River Laboratories International, Inc., a viral vector contract development and manufacturing organization (CDMO). Rznomics would leverage Charles River’s viral vector CDMO experience to initiate clinical development of its RNA-based anticancer gene therapy in liver cancer patients.
- In March 2023, LEO Pharma and ICON plc announced a strategic partnership that will enable LEO Pharma to scale clinical trial execution in a patient-centric and cost-effective way. The partnership would also support LEO Pharma’s overall ambition of building one of the most effective and efficient clinical portfolio execution organizations in the industry.
- In February 2023, Syneos Health partnered with Haystack Health, a Roivant Health portfolio company engaged in developing advanced artificial intelligence (AI) and natural language processing (NLP) solutions to improve the identification and enrollment of patients for clinical trials.
- In February 2023, Parexel launched a new expert series, New Medicines, Novel Insights. The series features fresh insights from the company’s global, cross-functional experts analyzing drug development trends and offering evidence-based guidance to the biopharmaceutical industry.
Competitive Landscape and Key Companies:
IQVIA Holdings Inc, Parexel International Corp, IXICO Plc, Charles River Laboratories International Inc, ICON Plc, WuXi AppTec Co Ltd, SGS SA, Syneos Health Inc, SIRO Clinpharm Pvt Ltd, Thermo Fisher Scientific Inc, and Laboratory Corp of America Holdings are among the major clinical trials companies in the global market. These companies have been implementing various strategies that have helped their growth and, in turn, have brought about various changes in the market. The companies have utilized organic strategies such as launches, expansions, and product approvals and inorganic strategies such as product launches, partnerships, and collaborations.

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Study Design, Phase, and Indication

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Frequently Asked Questions
The clinical trials market is analyzed on the basis of study design, phase, indication. Based on study design, the segment is segmented into interventional, observational, and expanded access. The interventional segment held the largest market share in 2022, and the same is anticipated to register the highest CAGR during the forecast period.
Clinical trials are research studies that test a medical, surgical, or behavioral intervention in people. These trials are the primary way that researchers determine if a new form of treatment or prevention, such as a new drug, diet, or medical device (for example, a pacemaker), is safe and effective in people. Often, a clinical trial is designed to learn if a new treatment is more effective or has less harmful side effects than existing treatments.
The factors that are driving the growth of the market are growth are increasing adoption and outsourcing of clinical trials, flourishing pharmaceutical industry and increasing R&D activities in pharmaceutical industry propelling market growth.
The clinical trials market majorly consists of the players such IQVIA Holdings Inc, Parexel International Corp, IXICO Plc, Charles River Laboratories International Inc, ICON Plc, WuXi AppTec Co Ltd, SGS SA, Syneos Health Inc, SIRO Clinpharm Pvt Ltd, Thermo Fisher Scientific Inc, and Laboratory Corp of America Holdings.
The List of Companies - Clinical Trials Market
- IQVIA Holdings Inc
- Parexel International Corp
- IXICO Plc
- Charles River Laboratories International Inc
- ICON Plc
- WuXi AppTec Co Ltd,
- SGS SA
- Syneos Health Inc
- SIRO Clinpharm Pvt Ltd
- Thermo Fisher Scientific Inc
- Laboratory Corp of America Holdings
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
Trends and growth analysis reports related to Clinical Trials Market

Jul 2023
Aquatic Veterinary Market
Size and Forecast (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Diagnostics and Treatments), Species (Fish, Crustaceans, Mollusca, and Others), Disease Source (Bacteria, Viruses, Parasites, and Others), Route of Administration (Water Medication, Medicated Feed, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Jul 2023
Sickle Cell Disease Treatment Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis By Treatment (Generic Drugs and Originators), Route of Administration (Oral and Parenteral), and Distribution Channel (Direct Tender, Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, and Others)

Jul 2023
Malaria and Sickle Cell Disease Treatment Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Treatment [Malaria and Sickle Cell Disease (SCD)], Route of Administration (Oral and Parenteral), and Distribution Channel (Direct Tender, Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, and Others)

Jul 2023
Pharmaceutical Contract Sales Organizations Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Services (Commercial Services and Non-Commercial Services), Modules (Syndicated Modules and Dedicated Modules), Therapeutic Area (Cardiovascular Disorders, Oncology, Metabolic Disorders, Neurology, Orthopedic Diseases, Infectious Diseases, and Others), End User (Biopharmaceutical Companies and Pharmaceutical Companies), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Jul 2023
Breast Cancer Therapeutics Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Drug Therapy [Targeted Drug Therapy (Abemaciclib, Ado-Trastuzumab Emtansine, Palbociclib, Trastuzumab, and Other Target Drug Therapies), Hormonal Drug Therapy (Selective Estrogen Receptor Modulators, Aromatase Inhibitors, and Selective Estrogen Receptor Downregulators), Chemotherapy, and Immunotherapy/Biological Therapy], Breast Cancer Type (Hormone Receptor, HER2+, and Triple-Negative Breast Cancer), Distribution Channel (Hospital Pharmacies, Drug Stores and Retail Pharmacies, and Online Pharmacies), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)

Jul 2023
Radioactive Tracer Market
Size and Forecast to 2030 - Global Analysis by Tracer Type [Technetium-99m & Tc-97m, Iodine-131, Iron-59, Lutetium-171, Rubidium (Rb-82) Chloride & Ammonia (N-13), Scandium-46, Seaborgium-269, Hassium-269, Gallium Citrate Ga 67, Prostate-Specific Membrane Antigen (PSMA) (Ga-68), FDDNP (F-18) & FDOPA (F-18), Phosphorus-32 & Chromium-51, Thallium-201, F-18 FDG, F-18 FAPI, Ga-68 FAPI, F-18 PSMA, DOTATOC/DOTANOC/DOTATATE (Ga-68), and Others], Test Type (PET, SPECT, and Others), Application (Oncology, Pulmonary, Neurology, Cardiology, and Others) End User (Hospitals & Clinics, Diagnostic Centers, Academic & Research Institutes, And Others), and Geography

Jul 2023
Glioma Treatment Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Disease (Astrocytoma, Oligoastrocytoma, and Oligodendroglioma), Treatment Type (Surgery, Chemotherapy, Radiation Therapy, and Others), Grade (Low Grade and High Grade), End User (Hospital & Clinics and Ambulatory Surgical Center), and Geography

Jul 2023
Hyperpigmentation Disorder Treatment Market
Size and Forecast (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Treatment Type (Cosmeceutical, Light or Laser Therapy, Microdermabrasion, Chemical Peels, Cryotherapy, and Others), Condition (Melasma, Solar Lentigines, Post-Inflammatory Hyperpigmentation, and Others), End User (Hospitals, Dermatology Centers, and Others), and Geography (North America, Europe, Asia Pacific, South & Central America, and Middle East & Africa)