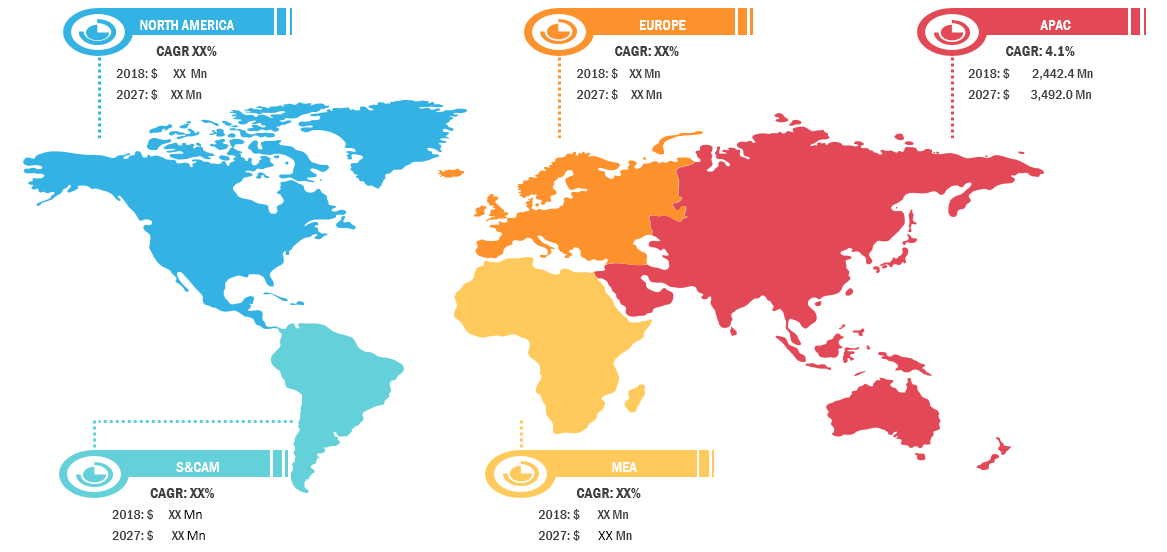
The global Antifungal Drugs market accounted to US$ 10,182.3 Mn in 2018 and is expected to grow at a CAGR of 3.2% during the forecast period 2019
The Antifungal drugs is an emerging market with consisting of the good and countable number of players, having considerable revenue in the market. Most of the companies operating in the global antifungal drugs market offers antifungal drugs to provide relief from various fungal infections.
The most notable Antifungal Drugs Market participants are Pfizer Inc., Sanofi S.A., Gilead Sciences, Inc., Merck And Co., Inc., Scynexis Inc., Novartis International AG, Abbott Laboratories, Bayer AG, Glenmark Pharmaceutical, and Glaxosmithkline Plc. among the others have occupied a considerable share of the market owing to their product offerings to the companies.
Market leaders are involved in organic and inorganic growth strategies in the antifungal drugs market. For instance, the companies have maximized their growth with several inorganic strategies to enhance the market value and position in the Antifungal Drugs Market. The inorganic developments in the market were maximum among the development in the antifungal drugs.
The market has experienced few organic strategies such as product launch, product approval and expansion activities that also has resulted positively for the growth of the Antifungal Drugs Market. The companies have also conducted lot of inorganic strategies such as partnership, acquisitions and agreements that has enhance the product portfolio.
Below is the list of the inorganic and organic strategies done by the players operating in the antifungal drugs market:
| Year | News | Region |
| May, 2019 | Merck and P&G signed an agreement to sale 51.80% stake of Merck Limited to P&G promoters at value of INR 1,290.19 crore. According to the agreement, Merck would be renamed as Procter and Gamble Health Ltd | Asia Pacific |
| September, 2018 | Aurobindo’s acquired generic dermatology portfolio of Novartis US which would include a wide range of therapeutic areas, including topical antibiotics, gynaecological and dermatological anti-fungal agents, anti-acne agents, local anaesthetic analgesics, anti-itch, and a dermatological chemotherapeutic agent. | North America |
| November, 2017 | Merck & Co., Inc. declared that the U.S. Food and Drug Administration (FDA) approved PREVYMIS (letermovir) to be uses as once-daily tablets for oral use and injection for intravenous infusion. | North America |
.