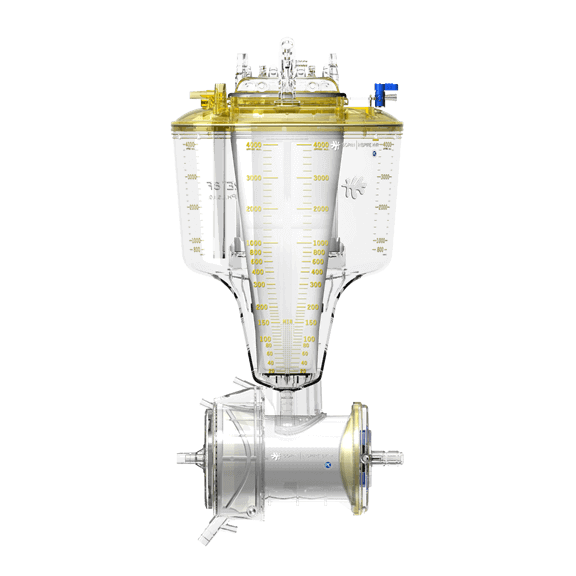
The oxygenator removes carbon dioxide and adds oxygen to the blood that is pumped into the arterial system. The blood pumped back into the patient’s arteries is sufficient to maintain life at even the most distant parts of the body as well as in the organs. Cardiopulmonary bypass (CPB) procedures require a blood-gas exchanger (oxygenator) to temporarily replace the respiratory function of the lungs. In the past the majority of CPB procedures have been carried out with bubble oxygenators which effect gas exchange by dispersion of bubbles into the blood. Membrane oxygenators, on the other hand, utilize a hydrophobic gas permeable membrane between the blood and gas phases. Bubble oxygenators are being superseded by membrane types for CPB due to improvements in membrane technology and mass transfer efficiency.
Getinge AG and Medtronic PLC – Notable Market Players in Cardiopulmonary Oxygenator Market
The cardiopulmonary oxygenator market comprises top players such as Getinge AB, LivaNova Plc, Medtronic Plc, Terumo Corp, Eurosets SRL, Nipro Medical Corp, Fresenius Medical Care AG & Co. KGaA, MicroPort Scientific Corp, Braile Biomédica Industry, Commerce and Representations Ltd, and Chalice Medical Ltd..
The companies listed above are implementing various strategies that have resulted in the company’s growth and, in turn, have brought about various changes in the worldwide market. Additionally, the companies have adopted several inorganic and organic strategies for accelerating their growth and improving their market position.
Below is the list of the growth strategies done by the players operating in the cardiopulmonary oxygenator market:
| Year | News |
| Nov-2021 | The Vitasprings Spiral Diversion Integrated Membrane Oxygenator (Vitasprings), a subsidiary of MicroPort Scientific Corporation (MicroPort), was given access by the National Medical Products Administration (NMPA) of China to enter the special approval procedure for innovative medical devices, also known as the ‘Green Path’. |
| Jan-2021 | Chalice Medical announced that the Paragon Maxi PMP oxygenator was approved for clinical use for up to 15 days. The Paragon Maxi PMP is the first 8 Litre polymethylpentene oxygenator to have been approved for use for upto 15 days. |
| Dec-2020 | Eurosets expanded its business by establishing its new facility in the UK. Eurosets UK Ltd provides full range of EUROSETS Cardiopulmonary and ECMO products directly from the United Kingdom. All existing and future products will be warehoused in the UK. |