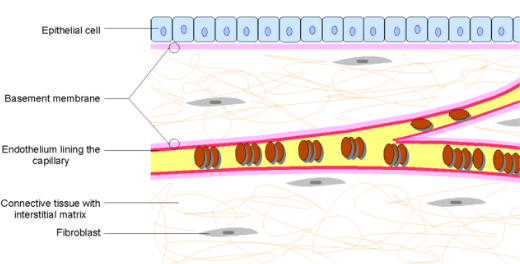
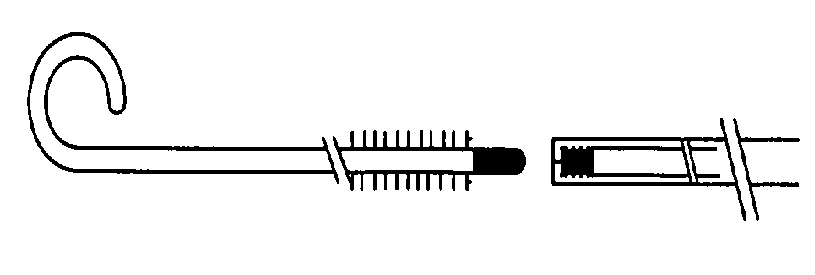
Micro coil or Embolization coils are the minimally invasive devices to diagnose and treat a patient from within a blood vessel. The process is done using continual X-ray visualization and high-speed radiographic filming techniques. After the procedure, the catheters are removed, patients are transferred to Intensive Care for monitoring and further care. These micro coils are made of soft platinum metal, Platinum Tungsten Alloy, and Platinum & Hydrogel and are shaped like a spring.
Terumo Corporation, Boston Scientific Corporation, Cook Medical LLC. – Notable Market Players in Micro coil Market
Market leaders operating in the market have undertaken various organic growth strategies in the micro coil market. The micro coil market majorly consists of the players including Terumo Corporation, Medtronic, Boston Scientific Corporation, Stryker Corporation, Cook Medical LLC, Penumbra, Inc., Balt USA LLC, Johnson and Johnson Services, Inc., Classic Coil Company, and KANEKA CORPORATION, among others. The companies have been implementing various strategies that have helped the growth of the company and in turn have brought about various changes in the market. The companies have utilized organic strategies such as new product launches, expansion, and product approvals.
Below is the list of the growth strategies done by the players operating in the micro coil market:
| Month & Year | News |
| Apr-2020 | Kaneka Corporation has received United States’ Food and Drug Administration approval for new micro coil i-ED Coil used in treatment of embolization of brain aneurysms. Kaneka plans to also expand the sales area to Europe and Asia and is aiming for 10 billion yen in sales of i-ED Coil by 2023. |
| May-2019 | Stryker has received premarket approval for Neuroform Atlas Stent System from US FDA. Neuroform Atlas Stent System consists of Target XL Detachable Coil for the treatment of wide-neck, intracranial aneurysms. |
| May-2018 | MicroVention, Inc., a U.S.-based subsidiary of Terumo received FDA Premarket Approval (PMA) for the LVIS and LVIS Jr. stents for stent-assisted coil embolization of intracranial aneurysms. The LVIS and LVIS Jr. stents are the first and only stents PMA approved for stent-assisted coil embolization and only the second PMA approved device designed for intracranial aneurysm treatment. |
| May-2019 | Stryker has received premarket approval for Neuroform Atlas Stent System from US FDA. Neuroform Atlas Stent System consists of Target XL Detachable Coil for the treatment of wide-neck, intracranial aneurysms. |