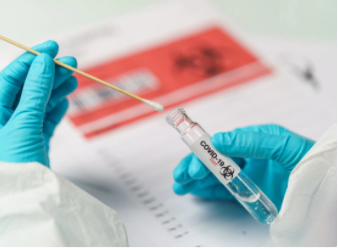
Swab collection kits are individually wrapped sterile swabs for biomedical research or clinical diagnostic uses. Swabs can be used in a variety of environments and specimen collection applications such as medical, forensics, genetics, microbiology, pre-analytical, and diagnostics.
BD and Puritan Medical Products Company LLC — Notable Market Players in Swabs Collection Kit Market
Leading players operating in the swabs collection kit market are adopting various growth strategies such as product launches, approvals, partnerships, and business planning. These strategies help them expand their business in the global market. BD, Puritan Medical Products, Hardy Diagnostics, LabCorp, Formlabs are among the leading companies operating in the market.
A few growth strategies adopted by the players operating in the swabs collection kit market are listed below:
| Month and Year | Description |
| June-2021 | BD (Becton, Dickinson and Company) a leading global medical technology company, announced the U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) for its rapid antigen test to be used for SARS-CoV-2 screening through serial testing of asymptomatic individuals. |
| March-2020 | LabCorp (NYSE: LH), a leading global life sciences company, announced that the US Food and Drug Administration (FDA) had granted Emergency Use Authorization (EUA) for its Pixel by LabCorp COVID-19 PCR Test Home Collection Kit for ages 2–17. The authorization expands the use of Pixel by LabCorp to children and adolescents two years of age and older when purchased by a parent or guardian. |
| December-2020 | HHSnd the Department of Defense (DoD) recently awarded a US$ 11.6 million contract to Puritan Medical Products Company, LLC to boost the domestic production of nasal swabs used for COVID-19 tests. Specifically, the contract will allow Puritan Medical to increase the production capability of Cue’s nasal swabs to 3 million per month by March 2021 at its Maine facility. HHS and DoD have continuously addressed the growing demand for testing supplies while also supporting facility capacity and vaccine allocation in the US. In April, 2020 the federal government awarded a US$ 75.5 million contract to Puritan to increase the company’s capacity to manufacture non-proprietary foam specimen collection swabs to 20 million swabs per month by July-2020. Then to further this effort, HHS and DoD awarded US$ 51.15 million to Puritan Medical to expand the industrial production capacity of flock tip testing swabs at its Maine location at the end of July-2020. |
| April-2020 | Hardy Diagnostics exponentially increased its manufacturing production to supply customers with critical laboratory products during the COVID-19 pandemic. Specifically, Hardy had increased production of Viral Transport Medium (VTM, Cat. No. R99), a product recommended by the CDC for collection and transportation of specimens from patients suspected of a COVID-19 infection. |
| March-2020 | Formlabs launched its 3D Printed Test Swabs for COVID-19 Testing in March 2020. These swabs are typically used for testing for influenza and other respiratory infections. The current and impending supply chain shortages are serious enough that clinicians are designing and testing their swabs as quickly and safely as possible. |