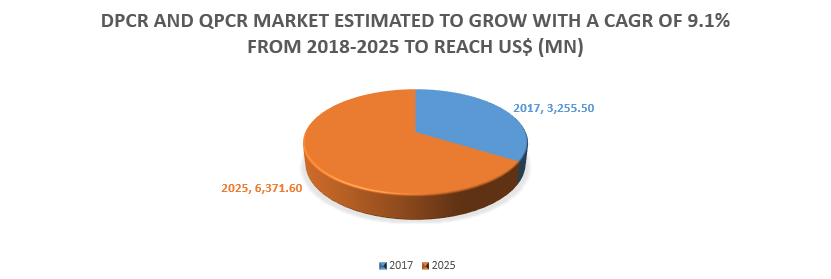
In-vitro diagnostics tests are performed on the samples such as blood, plasma, urine, and tissues for detection of diseases. The market is highly consolidated as smaller number of companies hold major share of the market. In-vitro diagnostics includes certain technologies such as molecular diagnostics, immunochemistry, clinical chemistry, and others. Increasing prevalence of chronic conditions are projected to drive the growth of the market.
The most notable market participants are F. Hoffmann-la Roche Ltd., Danaher, Abbott, Siemens AG, Sysmex Corporation, Thermo Fisher Scientific, Inc., BD, Biomérieux SA, Bio-Rad Laboratories, INC., and Qiagen, occupying a considerable share of the market owing to their offerings to the market.
F. HOFFMANN-LA ROCHE LTD., Abbott and Danaher Industries – Notable Market Participants in In-Vitro Diagnostics Industry
Market leaders are involved in offering the products and systems that contribute to quality enhancement and cost efficiency within healthcare and life sciences. For instance, in November 2015 Roche launched an in vitro diagnostic instrument namely VENTANA HE 600 system. The newly launched product is fully automated hematoxylin and eosin (H&E) tissue staining system which enhances patient and technician safety and produces exceptional staining quality.
Prominent players in in-vitro diagnostics market are focusing on organic strategies such as product launch, market expansion, and others. These strategies are helping major players to strengthen their market positions along with geographical expansion. Additionally various companies are also undergoing other strategic alliances such as acquisitions and others to garner their significance and remain competitive in the market. Few of the important key developments from the industry are mentioned below:
|
Year |
News |
Region |
|
2021 |
Roche announced that it has launched the two CE-IVD, which are its automated digital pathology algorithms, uPath HER2 (4B5) image analysis and uPath Dual ISH image analysis for breast cancer. It is likely to help in determining the best treatment strategy for each patient. The image analysis algorithms uses artificial intelligence to support pathologists in making faster, more accurate patient diagnoses in breast cancer. |
North America |
|
2021 |
Thermo Fisher Scientific Inc. announced that it has entered into a definitive agreement to acquire Mesa Biotech, Inc., a molecular diagnostic company. The acquisition will be for approximately US$ 450 million in cash. Under the agreement, Thermo Fisher Scientific Inc. will pay up to an additional US$ 100 million in cash after the completion of certain milestones following the close of the transaction. |
North America |
|
2020 |
bioMérieux SA announced receiving of first-ever COVID-19-related Certificate of Validation for its VERIPRO SARS-CoV-2 Environmental Assay (PTM 122001) by the AOAC INTERNATIONAL’s Emergency Response Validation (ERV) program. |
North America |
|
2019 |
QIAGEN launched the first FDA-approved companion diagnostic for PIK3CA biomarkers to enhance precision medicine in breast cancer. |
North America |
|
2018 |
Becton, Dickinson and Company, launched a molecular test for detection tuberculosis and multi-drug resistant tb, named as max mdr-tb. |
Europe |
|
2016 |
BioMérieux launched an in vitro diagnostic product namely EMAG which will provide functionality on molecular biology platform for the extraction of nucleic acids (DNA, RNA). |
Europe |