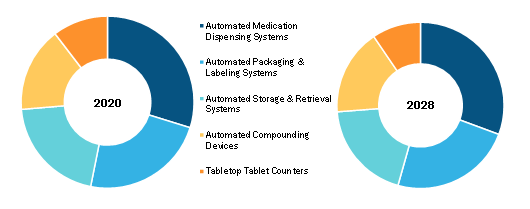
Companies in the pulmonary function testing system market are adopting developmental strategies such as product launches and mergers & acquisitions to sustain the competitive environment in the market. For instance, in January 2021, Vyaire Medical announced the European launch of ArtiQ. PFT, is in automated tool driven by artificial intelligence that instantly interprets pulmonary function tests (PFTs). ArtiQ. PFT is the first cloud-based PFT solution that leverages artificial intelligence. Similarly, in January 2023, CAIRE Inc., the leading global manufacturer of oxygen therapy and on-site generation systems, acquired MGC Diagnostics Holdings, Inc. (MGC). This acquisition has strengthened CAIRE’s position and focus on diagnostic technologies — further expanding its expertise in serving patients throughout the progression of pulmonary disease. Pulmonary function testing system companies across the world are scaling up their production. Their involvement in various business developments and product innovations helps ensure the easy availability of the products in the regional and global markets. Moreover, the regulatory agencies are providing the required support through timely product approvals and permissions.
Vyaire Medical and MGC Diagnostics – are Notable Market Players in Pulmonary Function Testing System Market
The pulmonary function testing system market majorly consists of the players such as Chest M.I Inc, Clarity Medical Pvt. Ltd., Cosmed srl, Eco Medics AG, Geratherm Medical AG, Medical Electronic Construction, MGC Diagnostics Corporation, Morgan Scientific Inc, NDD Medical Technologies Inc, Pulm One Advanced Medical Devices Ltd., Schiller AG, Koko LLC, and Vyaire Medical Inc. among others. The companies have been implementing various strategies that have helped the growth of the company and in turn have brought about various changes in the market. the companies have utilized organic strategies such as launches and product approvals. whereas, inorganic strategies such as mergers & acquisitions and collaborations, were widely seen in the pulmonary function testing system market.
Several in organic approaches, such as product launches, and expansion in the pulmonary function testing system market, have resulted in the positive growth of the market. Likewise, inorganic strategies such as mergers & acquisitions, and collaboration have helped the company to strengthen its revenue, which allows the company to hold a strong position in the market.
Below is the list of the growth strategies done by the players operating in the pulmonary function testing system market:
|
YEAR |
NEWS |
|
Jun-2022 |
ndd Medical Technologies (ndd) has updated the entire EasyOne product range, including the EasyOne Air, Easy on-PC, EasyOne Pro, and EasyOne Pro LAB, to be compliant with the ATS/ERS Standardization of Spirometry 2019. |
|
Apr-2021 |
Vyaire Medical announced the European launch of ArtiQ.PFT, an automated tool driven by artificial intelligence that instantly interprets pulmonary function tests (PFTs). ArtiQ.PFT is the first cloud-based PFT-solution that leverages artificial intelligence. |
|
Jan-2023 |
CAIRE Inc., the leading global manufacturer of oxygen therapy and on-site generation systems, acquired MGC Diagnostics Holdings, Inc. (MGC). This acquisition has strengthened CAIRE’s position and focus on diagnostic technologies — furthering expanding its expertise in serving patients throughout the progression of pulmonary disease. |
|
May-2022 |
MGC Diagnostics entered into a global distribution agreement with BedFont Scientific Ltd. to leverage its cardiopulmonary products and diagnostic device experience by providing Fractional Exhaled Nitric Oxide (FeNO) testing to measure airway inflammation for the management and aid in diagnosis of conditions such as asthma. |
|
Mar-2022 |
KoKo, LLC and MAGNET GROUP, signed a three-year partnership contract through September 14, 2024. The agreement allows MAGNET GROUP members to access to all KoKo’s respiratory and pulmonary function testing (PFT) products. |
|
Jul-2021 |
The partnership between KoKo, LLC and RBC Medical Innovations contributed to the ongoing efforts by focusing on expanding the manufacturing of diagnostic pulmonary function testing equipment. |
|
Aug-2020 |
ndd Medical Technologies announced its new partnership with Biomedix, a provider of front-line diagnostic solutions for delivering value-based care. Biomedix offers various platforms that centralize data for analyzing population health across multiple chronic conditions, including COPD. |
|
Mar-2020 |
MGC Diagnostics entered into partnership with Medical Specialties Inc. to expand their reach to all parts of the country and to promote their line of cardiorespiratory diagnostic instruments (Platinum Elite body plethysmographs and Ultima Series cardiorespiratory diagnosotic systems), Electronic Medical Record Integration (HL7 interface software and physician review software), and related services and supplies. |
|
Jan-2023 |
CAIRE Inc., the leading global manufacturer of oxygen therapy and on-site generation systems, acquired MGC Diagnostics Holdings, Inc. (MGC). This acquisition has strengthened CAIRE’s position and focus on diagnostic technologies — furthering expanding its expertise in serving patients throughout the progression of pulmonary disease. |
|
May-2022 |
MGC Diagnostics entered into a global distribution agreement with BedFont Scientific Ltd. to leverage its cardiopulmonary products and diagnostic device experience by providing Fractional Exhaled Nitric Oxide (FeNO) testing to measure airway inflammation for the management and aid in diagnosis of conditions such as asthma. |
|
Mar-2022 |
KoKo, LLC and MAGNET GROUP, signed a three-year partnership contract through September 14, 2024. The agreement allows MAGNET GROUP members to access to all KoKo’s respiratory and pulmonary function testing (PFT) products. |