Dermal Fillers Segment to Lead Aesthetic Fillers and Exosomes Market Based on Product Type During 2025–2031
According to our new research study on “Aesthetic Fillers and Exosomes Market Forecast to 2031 – Global Analysis – by Product Type, Route of Administration, Application, and End User,” the aesthetic fillers and exosomes market size is expected to grow from US$ 2,587.07 million in 2024 to US$ 4,642.07 million by 2031; the market is expected to register a CAGR of 8.8% during 2025–2031. Major factors driving the aesthetic fillers and exosomes market growth include the rise in demand for minimally invasive procedures, technological advancements, and product innovations, along with societal shifts toward youthful appearance and preventive care.
Aesthetic fillers, commonly known as dermal fillers, are injectables that are used to bring back the lost volume, get rid of wrinkles, and give the skin a smooth and refreshed appearance. Exosomes are extremely small structures secreted by cells to enable the cells near them to receive the same information. Personalized Aesthetic Treatments Powered by AI and Advanced Injectables are expected to be major aesthetic fillers and exosomes market trend in coming years.
Aesthetic Fillers and Exosomes Market, by Region, 2024 (%)
Aesthetic Fillers and Exosomes Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product Type [Dermal Fillers (Calcium Hydroxylapatite, Hyaluronic Acid, Collagen, Poly-L-Lactic Acid, Polymethylmethacrylate (PMMA), Fat Fillers, and Others) and Exosome-Based Aesthetic Products (Human-derived, Animal-derived, and Plant-derived)], Route of Administration [Injectable, Topical (Serum, Cream, Mask, and Others)], Application (Skin Rejuvenation, Wrinkle Reduction and Fine Line Correction, Facial Contouring, Lip Augmentation, Scar and Pigmentation, Post-procedure Recovery, Hair Restoration, and Others), End User (Hospitals, Dermatology Clinics, Medical Spas, and Others), and Geography (North America, Europe, and Middle East)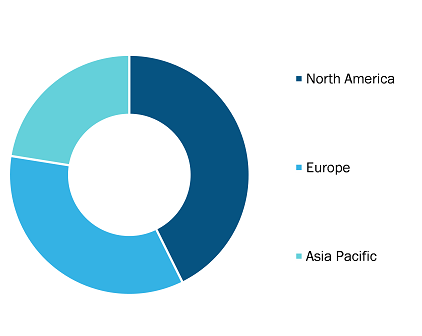
Aesthetic Fillers and Exosomes Market Size & Growth by 2031
Download Free Sample
Source: The Insight Partners Analysis
Aesthetic Fillers and Exosomes Market Analysis Based on Segmental Evaluation:
Based on product type, the aesthetic fillers and exosomes market is bifurcated into dermal fillers and exosome-based products. In 2024, the dermal fillers segment held a significant aesthetic fillers and exosomes market share. The term "dermal fillers" is associated with gel-based substances used to fill in wrinkles and furrows or increase the volume of areas such as the cheeks and lips. The difference between them lies only in the type of their component; they can be hyaluronic acid fillers, calcium hydroxylapatite fillers, poly-L-lactic acid fillers, polymethylmethacrylate fillers, or collagen-based fillers.
The skin produces hyaluronic acid that naturally keeps it hydrated and voluminous. The results are temporary and can last 6–12 months or even more until the body naturally absorbs the particles. The major FDA-approved hyaluronic acid fillers include Juvéderm products such as VOLBELLA, VOLLURE, Juvéderm XC, and VOLUMA; Restylane products such as Restylane, Restylane Refyne, Restylane Defyne, Restylane Kysse, Restylane Silk, Restylane Lyft, Restylane Contour; Belotero Balance; Revanesse Versa; and the RHA collection. The CaHA fillers are known for their longevity of effects and their potential to rejuvenate the skin by encouraging the production of collagen, which, in turn, results in increased elasticity and firmness. Radiesse is an injectable calcium hydroxylapatite dermal filler that is biocompatible and approved by the FDA to be used for the aesthetic augmentation of moderate to severe facial creases and folds. Poly-L-lactic acid fillers are synthetic polymers that are biodegradable and are currently being used as femal fillers for the gradual restoration of facial volume. The FDA-certified PLLA fillers are Sculptra and Sculptra Aesthetic. Polymethyl methacrylate fillers are a kind of permanent, non-biodegradable, and composed of PMMA microspheres in collagen gel. Likewise, collagen fillers work by refilling the skin with the help of collagen. They bring volume back to the areas of the skin that have been sunken, diminish the presence of scars, and provide the skin with a youthful look by helping smoothen facial wrinkles.
In August 2022, Allergan Healthcare India Private Limited launched Juvéderm VOLUX in India under the business unit of Allergan Aesthetics. It is a hyaluronic acid-based injectable implant designed to restore and enhance facial volume and is accompanied by lidocaine hydrochloride for patient comfort during the procedure. Moreover, Galderma received clearance from the US FDA for its new hyaluronic acid filler, Restylane Eyelight, in June 2023. It is used to correct under-eye hollows in adults.
The scope of the aesthetic fillers and exosomes market report includes an assessment of the market performance in North America, Europe, and the Middle East. In terms of revenue, North America dominated the aesthetic fillers and exosomes market share in 2024.
The US dominated the North America aesthetic fillers and exosomes market in 2024. The US is leading in the area of beauty fillers and exosome market, which is mainly caused by the culture of beauty and more than 6,000 cosmetic clinics in such places as Los Angeles and Miami. The injectable fillers based on hyaluronic acid are in the leading position, with almost 2 million treatments per year. They make up about 70% of all non-surgical interventions because of their short recovery time of 24–48 hours and their natural-looking results. Exosomes not only extend the duration of fillers but also provide 50% more collagen production and a reduction of the inflammatory process in the skin affected by the sun, which is the case in 75% of adults. On the other hand, exosomes enhance scar revision and hand rejuvenation by up to 45% skin texture improvement, and they make 95% of patients capable of resuming their normal activities, within 48 hours, quite possible.
Galderma SA; Merz Pharma GmbH & Co KGaA; Sinclair Pharma Ltd; TEOXANE LABORATORIES; Evolus Inc; IBSA Farmaceutici Italia Srl; BENEV COMPANY INC; Laboratorio Innoaesthetics, S.L.U.; Kimera Labs; Tiger Aesthetics Medical, LLC; Bioscience; Rion Aesthetics; Croma Pharma; and Exocel Bio are among the leading companies profiled in the aesthetic fillers and exosomes market report.
Based on product type, the aesthetic fillers and exosomes market is bifurcated into dermal fillers and exosome based products. Based on route of administration, the market is bifurcated into topical and injectable. By application, the market is segmented into skin rejuvenation, wrinkle reduction and fine line correction, facial contouring, lip augmentation, scar and pigmentation, post-procedure recovery, hair restoration, and others. By end user, the market is segmented into hospitals, dermatology clinics, medical spas, and others. In terms of geography, the market is categorized into North America (the US, Canada, and Mexico), Europe (France, Germany, the UK, Spain, Italy, and the Rest of Europe), and the Middle East (Saudi Arabia, the UAE, Turkey, Israel, and the Rest of Middle East).
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com