Fluorescent Immunoassay Market for ELISA Segment to Grow at Highest CAGR During 2021–2028
According to our new market research study on “Fluorescent Immunoassay Market to 2028 – Global Analysis and Forecast – by Product Type, Application, and End User,” the market is expected to grow from US$ 2,578.66 million in 2021 to US$ 3,778.78 million by 2028. The market is estimated to grow at a CAGR of 5.6% from 2021 to 2028. The report highlights trends prevailing in the market and drivers and hindrances pertaining to the market growth.
Based on product type, the global fluorescent immunoassay market is segmented into ELISA, rapid lateral flow immunoassay, and others. In 2021, the ELISA segment accounted for the largest share of the market. ELISA is a plate-based test technique for detecting and measuring soluble molecules such as peptides, proteins, antibodies, and hormones. The same method is also known by other names, such as enzyme immunoassay (EIA). An ELISA involves immobilizing the antigen (target macromolecule) on a solid surface (microplate) and then combining it with an antibody linked to a reporting enzyme. The reporter enzyme activity is measured by incubating it with a suitable substrate and producing a quantifiable result. A highly specific antibody-antigen interaction is the most important aspect of an ELISA. The significant use of immunoassay tests to diagnose cancer, infectious illnesses, and other chronic problems is responsible for the largest market share. The same segment is likely to register the highest CAGR in the global fluorescent immunoassay market during the forecast period.
In terms of application, the global fluorescent immunoassay market is segmented into infectious diseases, oncology, cardiovascular diseases, and others. The infectious diseases segment held the largest share of the market in 2021, and the same segment is anticipated to register the highest CAGR of 6.1% in the market during the forecast period.
Based on end user, the global fluorescent immunoassay market is segmented into hospitals & clinics, diagnostic centers, pharmaceutical & biotechnology companies, academic & research institutes, and others. The hospitals & clinics segment held the largest share of the market in 2021, and the same segment is anticipated to register the highest CAGR of 6.6% in the market during the forecast period.
The spread of COVID-19 severely affected medical systems in the region. Many commercially available tests for detecting SARS-CoV-2-specific antigens have recently been developed. The identification of COVID-19 antibodies in people as an alternative diagnostic method has recently received a lot of attention. To identify SARS-CoV-2, several clinics have used quick colloidal gold, enzyme-linked immunosorbent assay (ELISA), and chemiluminescence assays. These approaches, however, have flaws in terms of accuracy and sensitivity, with a large frequency of COVID-19 false positive and false negative reports. The STANDARD F COVID-19 Ag fluorescence immunoassay (FIA) kit is also available, which uses a fluorescent immunoassay to detect specific nucleoprotein antigens to SARS-CoV-2 in the human nasopharynx.
The development of a time-resolved fluorescence immunoassay (TRFIA) for detecting total COVID-19 antibodies in humans is the subject of a new study published in the journal Biotechnology and Applied Biochemistry. The authors of this study claim that TRFIA is a more sensitive and accurate approach for detecting the SARS-CoV-2 virus than the existing colloidal gold and chemiluminescence methods. The identification of antibodies is crucial in the diagnosis of COVID-19. To determine COVID-19 total antibodies, the manufacturers have developed a novel time-resolved fluorescence immunoassay (TRFIA). COVID-19 had positively impacted the global fluorescent immunoassay market as the technology was being used for COVID-19 diagnostics. Moreover, in May 2020, ERBA Diagnostics Mannheim GmbH (Germany) launched its immunoassay-based kit—ErbaLisa COVID-19 ELISA kits—to detect IgG and IgM antibodies against SARS-CoV-2. This kit enables the qualitative and semi-quantitative detection of IgG and IgM antibodies. In further research, the researchers improved the technique to increase the sensitivity and specificity of the strips for the inactivated viruses. In addition, researchers developed portable devices, reagents, and standards suitable for home use, similar to those used as a blood glucose meter.
Abbott, BD, bioMerieux SA, F. Hoffman-La Roche Ltd., Siemens AG, Sysmex Corp., Thermo Fisher Scientific Inc., Quidel Corp., Ortho Clinical Diagnostics Holdings plc, and Danaher are among the leading companies operating in the global fluorescent immunoassay market.
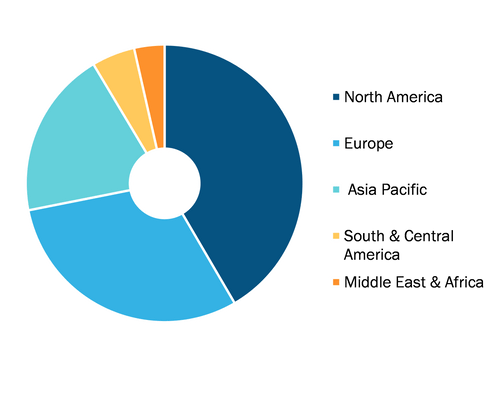
Global Fluorescent Immunoassay Market, by Region, 2021 (%)
Fluorescent Immunoassay Market Forecast and Size by 2031
Download Free Sample
Fluorescent Immunoassay Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product Type (ELISA, Rapid Lateral Flow Immunoassay, Others), Application (Infectious Diseases, Oncology, Cardiovascular Diseases, Others), End User (Hospitals & Clinics, Diagnostic Centers, Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others), and Geography
Fluorescent Immunoassay Market Forecast and Size by 2031
Download Free SampleFluorescent Immunoassay Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product Type (ELISA, Rapid Lateral Flow Immunoassay, Others), Application (Infectious Diseases, Oncology, Cardiovascular Diseases, Others), End User (Hospitals & Clinics, Diagnostic Centers, Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, Others), and Geography
By geography, the market is segmented into North America (the US, Canada, and Mexico), Europe (The UK, Germany, France, Italy, Spain, and the Rest of Europe), Asia Pacific (China, Japan, India, Australia, South Korea, and the Rest of Asia Pacific), the Middle East & Africa (UAE, Saudi Arabia, South Africa, and the Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and the Rest of South & Central America).
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com