Cancer Segment to Account for Maximum Share in Monoclonal Antibodies Market During 2021–2028
According to our latest study on “Monoclonal Antibodies Market Size and Forecast to 2028 – COVID-19 Impact and Global Analysis – by Source, Production Method, Indication, Application, End-User,” the monoclonal antibodies market is projected to reach US$ 243.05 billion by 2028 from US$ 111.01 billion in 2021; it is expected to grow at a CAGR of 11.8% from 2021 to 2028. The report highlights the key factors driving the market growth and prominent players with their developments in the market.
Based on indication, the monoclonal antibodies market is segmented into cancer, autoimmune diseases, infectious diseases, inflammatory diseases, microbial diseases, and others. The cancer segment is likely to account for a large market share during 2021–2028.
By source, the monoclonal antibodies market is segmented into human, humanized, chimeric, and murine. The human segment held a considerable share of the market in 2021 and is likely to continue its dominance in the market during the forecast period. The preventive administration of "palivizumab" human mAB proves effective against severe respiratory diseases that pose a high risk in infants and immunocompromised adults; it is the only mAB product licensed against infectious diseases. More such developments are expected in the market in the coming years, which are likely to drive the growth of the market for the human segment during 2021–2028.
The COVID-19 has significantly triggered the adoption of monoclonal antibodies in the last 2 years. A report by the NCBI states that the Food and Drug Administration (FDA) announced the emergency use authorization (EUA) for three monoclonal antibodies for the treatment of COVID-19 patients intended for treatment with the allocation of nearly 1 million monoclonal antibodies drugs. Also, monoclonal antibodies remain the only approved outpatient therapies intended for COVID-19 patients. Various regulatory agencies have introduced policies favoring the use of monoclonal antibodies in COVID-19 cases. For example, as per a report by the Crown, in August 2020, the Medicines and Healthcare products Regulatory Agency (MHRA) granted an approval for the first monoclonal antibody treatment intended for the prevention of COVID-19 in the UK.
Novartis AG; Pfizer Inc.; GlasxoSmithKline plc.; Amgen Inc.; DAICHI SANKYO COMPANY LIMITED; F. HOFFMANN-LA ROCHE LTD.; AstraZeneca; Elli Lilly and Company; Bayer AG; Bristol-Myers Squibb Company are among the leading companies operating in the monoclonal antibodies market.
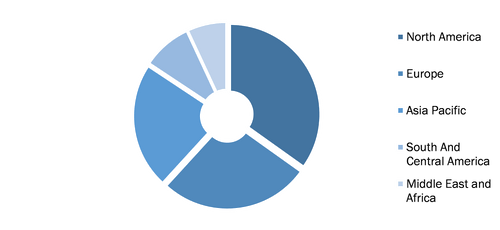
Monoclonal Antibodies Market, by Region, 2021 (%)
Monoclonal Antibodies Market Share & Demand Insights 2034
Download Free Sample
Monoclonal Antibodies Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Source (Human, Humanized, Chimeric, and Murine), Production Method (In-Vitro and In-Vivo), Indication (Cancer, Autoimmune Diseases, Infectious Diseases, Inflammatory Diseases, Microbial Diseases, and Others), Application (Therapeutic Applications, Diagnostic Applications, and Research Applications), and End-User (Hospitals, Others, and Research Institutes)
Monoclonal Antibodies Market Share & Demand Insights 2034
Download Free SampleMonoclonal Antibodies Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Source (Human, Humanized, Chimeric, and Murine), Production Method (In-Vitro and In-Vivo), Indication (Cancer, Autoimmune Diseases, Infectious Diseases, Inflammatory Diseases, Microbial Diseases, and Others), Application (Therapeutic Applications, Diagnostic Applications, and Research Applications), and End-User (Hospitals, Others, and Research Institutes)
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com