Product Segment to Contribute Larger Share to Nitinol Medical Devices Market during 2021–2028
According to the latest study on “Nitinol Medical Devices Market Forecast to 2028 – COVID-19 Impact and Global Analysis – by Product and Application,” the market was valued at US$ 15,807.53 million in 2020 and is projected to reach US$ 27,327.25 million by 2028; it is expected to grow at a CAGR of 7.2% during 2021–2028. The report highlights the trends prevailing in the market, and drivers and deterrents pertaining to the market growth.
Based on product, the nitinol medical devices market is segmented into nitinol stents, nitinol guidewires, nitinol filters, nitinol basket, nitinol catheters, and others. In 2020, the nitinol stents segment accounted for the largest share of the market. The market growth for this segment is attributed to the changing perception of customers toward the utilization of nitinol medical devices.
The factors such as the increasing adoption of minimally invasive surgeries (MIS) and growing prevalence of chronic diseases drive the growth of the nitinol medical devices market. However, manufacturing challenges restrain the market growth. On the other side, the formation of regulatory guidelines would offer lucrative opportunities for the growth of the market during the forecast period.
Cook Medical LLC; Abbott Laboratories; Zimmer Biomet Holdings, Inc; B. Braun Melsungen AG; Boston Scientific Corporation; Becton, Dickinson and Company (BD); Arthrex, Inc.; W. L. Gore & Associates, Inc; Terumo Corporation; and Nordson Corporation are among the prominent players operating in the nitinol medical devices market. These companies are adopting partnerships, product launches, collaborations, and other business strategies to sustain their position in the market. For instance, in February 2021, Cook Medical announced that the Approach Authority Workhorse Microwire Guide is now commercially available in the US and Canada. Approach Authority combines resilient nitinol tip technology with a hydrophilic coating. These wire guides can be used for various vascular procedures.
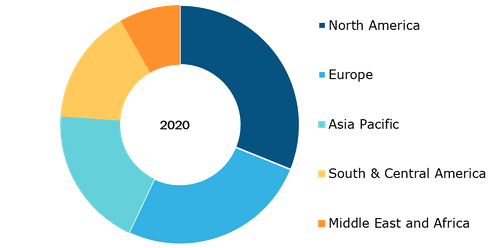
Nitinol Medical Devices Market, by Region, 2020 (%)
Nitinol Medical Devices Market Forecast and Size by 2031
Download Free Sample
Nitinol Medical Devices Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Nitinol Stents, Nitinol Guidewires, Nitinol Filters, Nitinol Basket, Nitinol Catheters, and Others ), Application (Orthopedic, Vascular, Dental, and Gastroenterology ), and Geography
Nitinol Medical Devices Market Forecast and Size by 2031
Download Free SampleNitinol Medical Devices Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Nitinol Stents, Nitinol Guidewires, Nitinol Filters, Nitinol Basket, Nitinol Catheters, and Others ), Application (Orthopedic, Vascular, Dental, and Gastroenterology ), and Geography
The report segments the global nitinol medical devices market as follows:
- Nitinol Stents
- Nitinol Guidewires
- Orthodontic Guidewires
- Endoscopic Guidewires
- Nitinol Filters
- Nitinol Basket
- Nitinol Catheters
- Others
- Orthopedic
- Vascular
- Dental
- Gastroenterology
By Geography
- North America
- US
- Canada
- Mexico
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia Pacific
- Middle East and Africa
- UAE
- Saudi Arabia
- South Africa
- Rest of Middle East and Africa
- South and Central America
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com