Tissue Engineering Segment to Lead Regenerative Medicine Market Based on Product Type During 2025–2031
According to our new research study on “Regenerative Medicine Market Forecast to 2031 – Global Analysis – by Product Type, Application,” the regenerative medicine market size is expected to grow from US$ 20.55 billion in 2024 to US$ 75.16 billion by 2031; the market is expected to register a CAGR of 20.3% during 2025–2031. Factors driving the regenerative medicine market growth include the rising prevalence of chronic disorders, an aging population, and the introduction of advanced regenerative therapies.
Regenerative medicine is a field of biomedical science focusing on repairing, replacing, or regenerating damaged or diseased cells, tissues, or organs to restore normal function. It combines approaches from stem cell therapy, tissue engineering, gene therapy, and biomaterials to promote healing and regeneration. Integration of Artificial Intelligence in Regenerative Medicine is expected to emerge as a significant regenerative medicine market trend in the future.
Regenerative Medicine Market, by Region, 2024(%)
Regenerative Medicine Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage:By Product Type [Cell-Based Products (Stem Cell and Cell-Based Immunotherapy), Gene Therapy, Tissue Engineering], Application (Oncology, Neurological Disorders, Wound Healing & Skin Regeneration, Ophthalmology, Orthopedics & Musculoskeletal, Immunology, Genetic Disorders, and Others), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)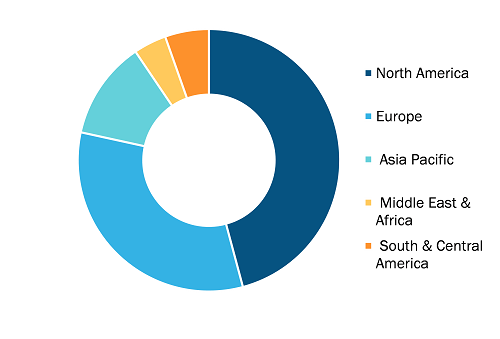
Regenerative Medicine Market Size, Share & Forecast 2025-2031
Download Free Sample
Regenerative Medicine Market Analysis Based on Segmental Evaluation:
Based on product type, the regenerative medicine market is segmented into cell-based products, gene therapy, and tissue engineering. In 2024, the tissue engineering segment held a significant regenerative medicine market share. Tissue engineering products encompass scaffolds, bio-matrices, skin grafts, cartilage and bone regeneration products, and advanced 3D bioprinted tissues and organs. These repair, regenerate, or replace damaged tissues and organs by providing biocompatible frameworks and living cells that support natural healing and regeneration processes.
In July 2021, 3DBioFibR, an innovator in tissue engineering, announced the release of two new products: CollaFibR (referred to as micro-CollaFibR) and the CollaFibR 3D scaffold. These off-the-shelf products are created using 3D BioFibR’s proprietary dry-spinning technology, which allows for the production of collagen fibers at a commercial scale. They offer advantages for tissue engineering and tissue culture applications and are now available for purchase by the scientific and medical communities. Globally, over 160 tissue engineering and regenerative therapeutic products have been developed by companies, many of which focus on applications in chronic wound care, orthopedics, and skin regeneration. Technological advances such as 3D bioprinting allow for more precise and cost-effective production of engineered tissues, broadening clinical application and acceptance.
The scope of the regenerative medicine market report includes an assessment of the market performance in North America, Europe, Asia Pacific, South and Central America, and the Middle East and Africa. In terms of revenue, North America dominated the regenerative medicine market share in 2024.
The regenerative medicine market in North America is segmented into the US, Canada, and Mexico. The US holds the largest market share, followed by Canada. According to the Centers for Disease Control and Prevention (CDC), a heart attack occurs every 40 seconds in the US, with approximately 805,000 people experiencing it each year. Cancer is a major cause of morbidity and mortality, with significant healthcare costs and quality-of-life implications. The rising prevalence of chronic and degenerative conditions is increasing the demand for novel treatment approaches such as regenerative therapies.
Regenerative medicine holds transformative potential for prevalent diseases in North America, particularly cardiovascular diseases, diabetes, and certain types of cancer. Advances in stem cell therapies, gene editing, and tissue engineering are showing promise in these areas. CAR-T cell therapies have received FDA approval for treating hematological malignancies, such as multiple myeloma and lymphoma.
The rising burden of chronic and degenerative diseases is driving investments in research, biomanufacturing, and workforce development within the regenerative medicine sector. This ecosystem fosters rapid innovation and enhances the availability and sophistication of treatment options. In January 2022, Cellino Biotech, Inc., a US-based autonomous cell therapy manufacturing company, completed a US$ 80 million Series A financing round led by Bayer AG's Leaps by Bayer, alongside 8VC and the Humboldt Fund. Cellino’s innovative manufacturing platform combines artificial intelligence and laser technology to automate cell therapy production, aiming to lower costs and improve scalability. This investment offers Leaps by Bayer an opportunity to support the development of advanced therapies that enhance patient access to cell treatments.
According to the CDC, in 2023, 76.4% of US adults, over 194 million people, had at least one chronic condition. More than half of these individuals (51.4%, or 131 million) reported having two or more chronic conditions. The prevalence of at least one chronic condition among adults increased from 72.3% to 76.4%. The percentage of adults with two or more chronic conditions rose from 47.3% to 51.4%.
According to the Alzheimer’s Association, by 2025, more than 7.2 million Americans aged 65 and older are projected to be living with Alzheimer’s, with that number expected to grow to 12.7 million by 2050. Chronic illnesses such as cardiovascular diseases, cancer, and neurodegenerative disorders lead to irreversible damage to tissues or organs, making patients prime candidates for regenerative therapies such as stem cell therapy, tissue engineering, and gene therapy. Regenerative medicine shows promise in restoring lost heart function after heart attacks, regenerating cartilage in cases of degenerative joint diseases, and replacing damaged neurons in neurodegenerative disorders. In the US, MSCs are used to treat degenerative diseases and certain skin, bone, and cartilage diseases.
The US government is promoting the development and approval of regenerative therapies by utilizing regulatory innovation, expedited approval processes, direct funding, and collaborative technical support. As of mid-2025, more than 80 Regenerative Medicine Advanced Therapy (RMAT) designations have been awarded, which have helped speed up clinical advancements for conditions such as spinal cord injuries and heart failure. In 2023, the US FDA approved cell and gene therapies, including gene-editing treatments targeting rare diseases. Therapies such as exagamglogene autotemcel (Casgevy) and lovotibeglogene autotemcel for sickle cell disease, as well as valoctocogene roxaparvovec for severe hemophilia A, have received FDA approval, highlighting the potential of gene therapies in addressing rare disease challenges. Thus, the increasing incidence of chronic diseases, coupled with ongoing research and approval initiatives to promote the adoption of regenerative technologies, is fueling the market growth in the US.
Bristol-Myers Squibb Co; Novartis AG; Johnson & Johnson; Daiichi Sankyo Co Ltd; Takeda Pharmaceutical Co Ltd; Japan Tissue Engineering Co., Ltd; Bluebird Bio Inc; JCR Pharmaceuticals Co. Ltd; Vertex Pharmaceuticals Inc; Ferring Pharmaceuticals; CSL Behring LLC; and BioMarin Pharmaceutical Incare among the leading companies profiled in the regenerative medicine market report.
Based on product type, the regenerative medicine market is segmented into cell-based products, gene therapy, and tissue engineering. By application, the regenerative medicine market is divided into oncology, neurological disorders, wound healing & skin regeneration, ophthalmology, orthopedics & musculoskeletal, immunology, genetic disorders, and others. Per geography, the market is categorized into North America (US, Canada, and Mexico), Europe (France, Germany, UK, Spain, Italy, and the Rest of Europe), Asia Pacific (China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific), the Middle East and Africa (Saudi Arabia, South Africa, UAE, and the Rest of Middle East and Africa), and South and Central America (Brazil, Argentina, and the Rest of South and Central America).
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com