Anesthesia Monitoring Devices Market Size and Forecast 2025-2031
Anesthesia Monitoring Devices Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product [Basic Anesthesia Monitors, Integrated Anesthesia Workstations, and Advanced Anaesthesia Monitors (Depth of Anaesthesia Monitors, Anaesthesia Gas Monitors, and Standalone Capnograph Monitors)], Technology [Bispectral Index (BIS), Auditory Evoked Potential (AEP), Patient State Index (PSI), Cortical Activity State Index (CSI), and Others], End User (Hospitals, Specialty Clinics, and Ambulatory Surgical Centers), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, South and Central America)
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Oct 2025
- Report Code : TIPHE100001202
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 286
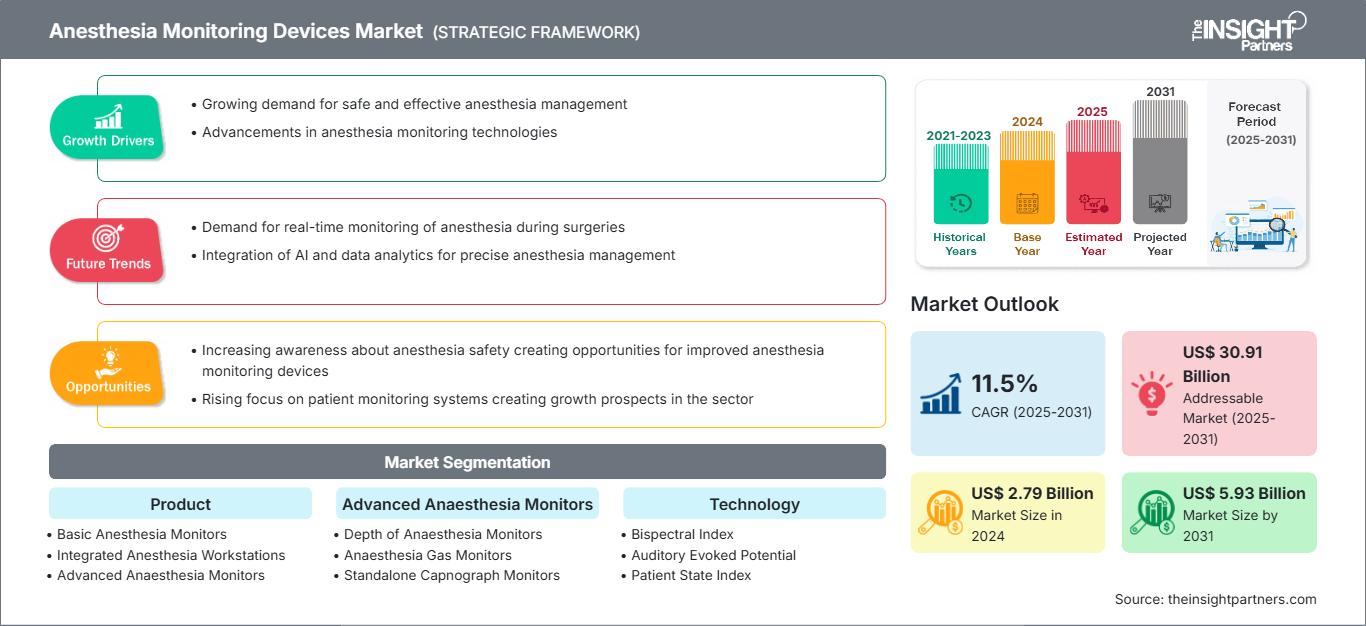
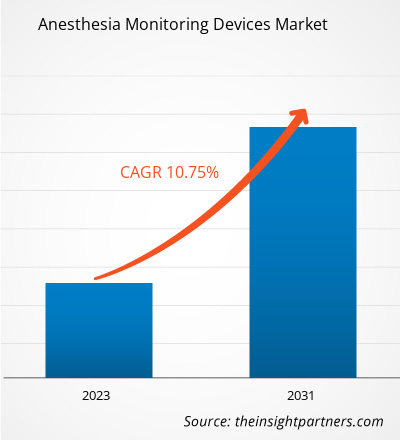
The anesthesia monitoring devices market size is expected to reach US$ 5.78 billion by 2031 from US$ 2.79 billion in 2024. The market is anticipated to register a CAGR of 11.1% during 2025–2031.
Anesthesia Monitoring Devices Market Analysis
The global anesthesia monitoring devices market continues to expand at a stable pace, reflecting the main trends such as the growing number of surgeries worldwide, increasing elderly population, and escalating need for the real-time monitoring of patients during anesthesia. A key driver of technological advancements is the enhanced performance and user experience achieved through advanced sensors, wireless connectivity, and touchscreen interfaces in modern devices. The adoption of anesthesia monitoring devices in the emerging markets is propelled by the rebuilding of healthcare infrastructure and improved accessibility to surgical care.
Anesthesia Monitoring Devices Market Overview
An anesthesia monitoring device plays a vital role in a hospital setting by helping anesthesiologists and surgical teams keep the patient safe and achieve good clinical results during the entire operative process. This advanced technology continuously collects patients' vital physiological parameters, including brief heart rate, blood pressure, blood oxygenation, breathing rate, and concentration of carbon dioxide at the end of exhalation. By providing accurate data, anesthesia monitors allow the healthcare staff to make accurate clinical decisions. Maintaining the patient's stable condition and optimizing the dose of anesthesia are essential throughout the surgery.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONAnesthesia Monitoring Devices Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Anesthesia Monitoring Devices Market Drivers and Opportunities
Market Drivers:
- Increasing Volume and Complexity of Surgical Procedures: The global rise in surgical procedures, both elective and emergency, is the leading cause pushing the demand for anesthesia monitoring devices.
- Tech-Enabled Transformation in Anesthesia Monitoring Systems: Technological innovation is changing the anesthesia monitoring devices market by significantly improving their capabilities and customer satisfaction.
- Elevated Focus on Patient Safety and Regulatory Compliance: Patient safety has become the most critical factor in healthcare provision and purchasing decisions, thus significantly boosting the anesthesia monitoring devices market.
Market Opportunities:
- Unlocking Market Potential in Developing Regions: The new markets of Asia Pacific, Latin America, and the Middle East and Africa are the growth horizons for the anesthesia monitoring devices market.
- Anesthesia Monitoring in Ambulatory and Minimally Invasive Settings: Ambulatory Surgery Centers (ASCs) and environments for minimally invasive procedures have become a new source of demand for anesthesia monitoring devices.
- Digital Transformation in Anesthesia Monitoring: The revolutionary combination of digital health technologies, artificial intelligence (AI), machine learning (ML), and the Internet of Things (IoT) is transforming the anesthesia monitoring field, opening up new growth opportunities.
Anesthesia Monitoring Devices Market Report Segmentation Analysis
The anesthesia monitoring devices market is divided into different segments to give a clearer view of how it works, its growth potential, and the latest trends. Below is the standard segmentation approach used in most industry reports:
By Product:
- Basic Anesthesia Monitor: Suitable for regular surgical operations, this device delivers the necessary monitoring parameters with cost-effective functionality.
- Integrated Anaesthesia Workstations: These machines simplify the patient's perioperative care by combining monitoring, ventilation, and anaesthesia delivery into a single, streamlined unit.
- Advanced Anesthesia Monitors: These devices allow for sophisticated monitoring and provide features such as gas analysis, depth of anesthesia tracking, and electronic health system integration for the management of critical and complicated surgeries.
By Advanced Anaesthesia Monitors:
- Depth of Anaesthesia Monitors: Follows the brain work to evaluate and adjust the anaesthesia level, thus minimizing the chances of under- or over-sedation
- Anaesthesia Gas Monitors: Measure the gases of anaesthesia that are inhaled and exhaled continuously to guarantee the exact dosing of the patient
- Standalone Capnograph Monitors: The real-time source of breath CO₂ concentration is an essential parameter for the correct respiratory function assessment during anesthesia.
By Technology:
- Bispectral Index (BIS): This method employs EEG signals to measure the level of sedation on a scale from 0 to 100. Thus, it is beneficial in determining the optimal dose of anaesthesia for each patient.
- Auditory Evoked Potential (AEP): The core of this method is the measurement of the brain's response to an auditory stimulus. The resulting record is used to assess the depth of anesthesia and evaluate the neurological function.
- Patient State Index (PSI): Patient State Index (PSI) is a consciousness index derived from EEG signals along with several other parameters. The aim of this index is to provide a guide for anesthetic management.
- Cortical Activity State Index (CSI): This method is based on EEG, which records cortical brain activity. The information obtained is used to assess and keep the correct depth of anaesthesia.
- Others: It includes new and alternative technologies for anaesthesia monitoring, such as non-publicly known indices and mixed EEG systems.
By End-User:
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
- Hospitals and Clinics
Each sector has specific anesthesia monitoring device requirements. It influences brain data management and analysis.
By Geography:
- North America
- Europe
- Asia Pacific
- South & Central America
- Middle East & Africa
The anesthesia monitoring devices market in Asia Pacific is expected to witness the fastest growth over the forecast period, driven by rapid healthcare digitalization in countries such as China, India, and Japan.
Anesthesia Monitoring Devices Market Regional InsightsThe regional trends and factors influencing the Anesthesia Monitoring Devices Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Anesthesia Monitoring Devices Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Anesthesia Monitoring Devices Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 2.79 Billion |
| Market Size by 2031 | US$ 5.78 Billion |
| Global CAGR (2025 - 2031) | 11.1% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Product
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Anesthesia Monitoring Devices Market Players Density: Understanding Its Impact on Business Dynamics
The Anesthesia Monitoring Devices Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Anesthesia Monitoring Devices Market top key players overview
Anesthesia Monitoring Devices Market Share Analysis by Geography
Asia Pacific is expected to grow the fastest in the next few years. Emerging markets in South and Central America and the Middle East and Africa also present many untapped opportunities for anesthesia monitoring device providers to expand.
The anesthesia monitoring devices market grows differently in each region due to factors such as healthcare infrastructure, regulatory environment, digital health adoption, and government initiatives. Below is a summary of market share and trends by region:
1. North America
Leads the anesthesia monitoring devices market due to advanced surgical infrastructure, strong digital health integration, and regulatory compliance2. Europe
Holds a substantial market share supported by well-established public health systems and stringent data governance like GDPR3. Asia Pacific
Fastest-growing market owing to rising surgical volumes, healthcare digitization, and expanding medical tourism4. South and Central America
An emerging market with growing adoption of digital monitoring tools in both public and private surgical centers5. Middle East and Africa
Developing region with high growth potential, driven by national e-health initiatives and expanding surgical infrastructureAnesthesia Monitoring Devices Market Players Density: Understanding Its Impact on Business Dynamics
High Market Density and Competition
Competition is intense due to the presence of major vendors such as GE HealthCare Technologies Inc. and Medtronic. Regional and niche players such as Schiller AG (Germany) also contribute to the market landscape.
This competitive environment pushes vendors to differentiate through:
- Advanced integration with hospital information systems (HIS) and electronic medical records (EMR)
- Compact, modular, and portable monitoring solutions suitable for both high-acuity and outpatient settings
Opportunities and Strategic Moves
- Collaborate with hospitals and surgical centers to enable smart ORs and digitized perioperative workflows
- Invest in research and development for noninvasive and real-time monitoring technologies, including wireless and wearable anaesthesia sensors
Other companies analyzed during the course of research:
- Masimo Corporation
- Nihon Kohden Corporation
- Spacelabs Healthcare (OSI Systems, Inc.)
- BPL Medical Technologies
- EDAN Instruments, Inc.
- Criticare Systems, Inc.
- Fukuda Denshi Co., Ltd.
- Smiths Medical Inc.
- Siemens Healthineers
- Teleflex Incorporated
- Ambu A/S
- Danmeter ApS
- Medasense Biometrics Ltd.
- Siare Engineering International Group s.r.l.
- Getinge AB (via MAQUET)
Anesthesia Monitoring Devices Market News and Recent Developments
- GE HealthCare (Nasdaq: GEHC) announced the publication of the MASTER trial results in the peer-reviewed journal Anesthesia and Analgesia, demonstrating the safety and efficacy of End-tidal Control software for inhaled anesthetic administration for surgical patients. End-tidal Control software automatically achieves and maintains clinician-set targets of end-tidal anesthetic agent and oxygen concentrations. The results highlight End-tidal Control’s performance in achieving and maintaining targeted agent and oxygen concentrations during anesthesia delivery compared to conventional manual control.
- SCHILLER extends its monitoring range with the MAGLIFE RT-1. The MAGLIFE RT-1 performs patient monitoring in an MRI environment, including all necessary vital parameters during anaesthesia. The system is designed for all patients: adults, children, and neonates. It allows for close monitoring during the examination and can be fully controlled from outside the Faraday cage.
Anesthesia Monitoring Devices Market Report Coverage and Deliverables
The "Anesthesia Monitoring Devices Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Anesthesia monitoring devices market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Anesthesia monitoring devices market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Anesthesia monitoring devices market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the anesthesia monitoring devices market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For