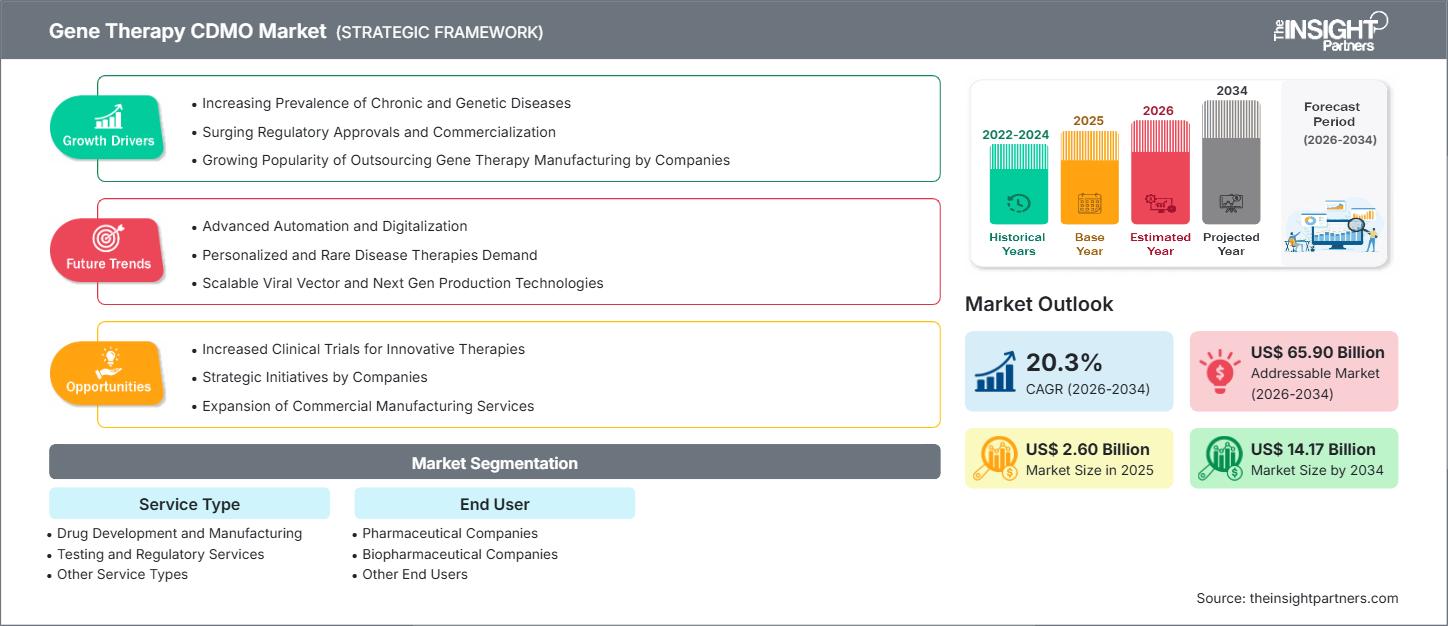
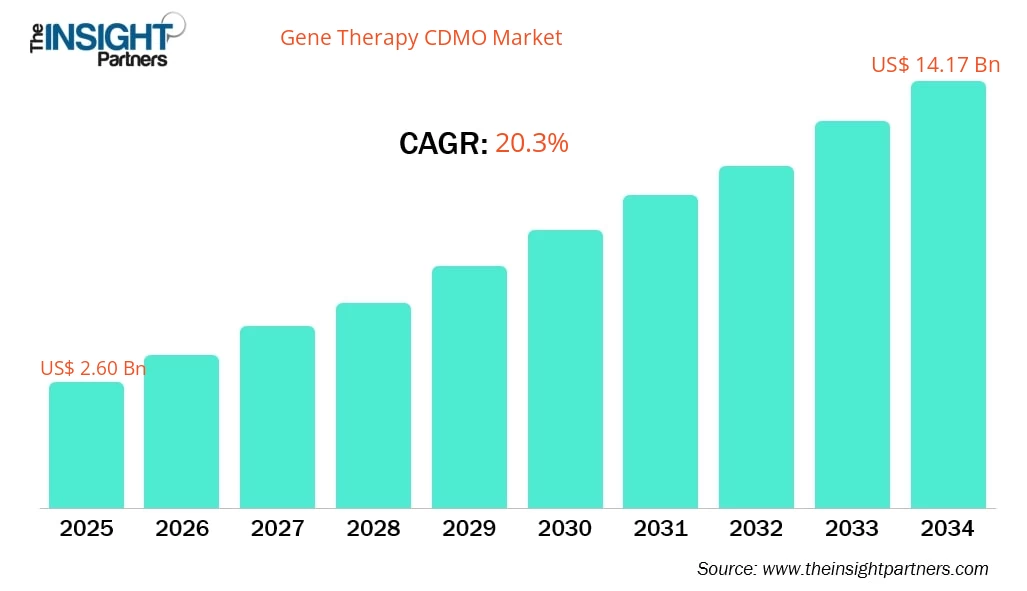
The Gene Therapy CDMO Market size is projected to reach US$ 14.17 billion by 2034 from US$ 2.60 billion in 2025. The market is expected to register a CAGR of 20.3% during 2026–2034.
Gene Therapy CDMO Market Analysis
The gene therapy CDMO market is experiencing robust growth due to large-scale outsourcing of manufacturing services, the use of technology to develop better vectors, the formation of strategic industry partnerships, and strong regulatory support. Additionally, the focus on rare and genetic diseases, along with the limited capacity of internal manufacturing facilities, drives biopharmaceutical companies to rely on specialized CDMOs for high-quality, scalable production solutions.
Gene Therapy CDMO Market Overview
The gene therapy CDMO market is growing at a rapid pace. Primary drivers of growth include an expanding research pipeline and the increasing acceptance of gene-based treatments across various disease areas. Although the market is crowded, companies can still offer strong services through process optimization, adoption of next-generation delivery platforms, and investment in flexible manufacturing facilities. Additionally, regulatory harmonization and incentives are driving innovation and accelerating development timelines. A strong focus on quality assurance, biosafety, and risk mitigation is leading the pharmaceutical industry to outsource complex production tasks. CDMOs are bringing in talent and expertise ideally suited for custom development needs, while strategically expanding geographically close to emerging markets.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONGene Therapy CDMO Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Gene Therapy CDMO Market Drivers and Opportunities
Market Drivers:
- Increasing Prevalence of Chronic and Genetic Diseases: Rising incidence of genetic and chronic disorders drives demand for innovative gene therapies, prompting biopharma firms to collaborate with CDMOs for efficient development and production.
- Surging Regulatory Approvals and Commercialization: Accelerated approvals and growing commercialization of gene therapies encourage manufacturers to scale production, fueling the expansion of CDMO services globally.
- Growing Popularity of Outsourcing Gene Therapy Manufacturing: Biopharma companies increasingly outsource complex gene therapy manufacturing to specialized CDMOs to reduce costs, access expertise, and ensure scalable, high-quality production.
Market Opportunities:
- Increased Clinical Trials for Innovative Therapies: The growing clinical trial pipeline for innovative gene therapies has increased the demand for contract manufacturing support, providing CDMOs with opportunities to expand in both early and late stages of development.
- Strategic Initiatives by Companies: Collaborations, partnerships, and mergers among gene therapy companies are major drivers of CDMOs’ service adoption, which in turn fosters innovation and expands market penetration.
- Expansion of Commercial Manufacturing Services: By increasing their commercial manufacturing capacity, CDMOs can meet rising global demand, thereby facilitating large-scale gene therapy distribution and driving market growth.
Gene Therapy CDMO Market Report Segmentation Analysis
The gene therapy CDMO market is segmented into distinct categories to provide a clearer understanding of its operations, growth potential, and current trends. Below is the standard segmentation approach used in industry reports:
By Type:
- Drug Development and Manufacturing: The growing demand for high-quality and scalable gene therapy production is the primary reason drug development and manufacturing services are gaining traction. The biopharma industry is increasingly relying on CDMOs with expertise in handling these complex processes.
- Testing and Regulatory Services: Stringent regulatory requirements, along with the need for rigorous safety and quality testing, drive the growth of testing and regulatory services, ensuring therapies efficiently meet compliance standards.
- Other Service Types: The demand for support services—such as formulation, filling, and supply chain management—is increasing, as CDMOs offer comprehensive solutions that help clients reduce time-to-market and operational complexities.
By End User:
- Pharmaceutical Companies
- Biopharmaceutical Companies
- Other End Users
By Geography:
- North America
- Europe
- Asia Pacific
- South and Central America
- Middle East and Africa
Gene Therapy CDMO Market Regional Insights
The regional trends and factors influencing the Gene Therapy CDMO Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Gene Therapy CDMO Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Gene Therapy CDMO Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2025 | US$ 2.60 Billion |
| Market Size by 2034 | US$ 14.17 Billion |
| Global CAGR (2026 - 2034) | 20.3% |
| Historical Data | 2021-2024 |
| Forecast period | 2026-2034 |
| Segments Covered |
By Service Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Gene Therapy CDMO Market Players Density: Understanding Its Impact on Business Dynamics
The Gene Therapy CDMO Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Gene Therapy CDMO Market top key players overview
Gene Therapy CDMO Market Share Analysis by Geography
The market is expected to grow at the fastest rate in Asia Pacific over the next few years. Emerging markets in South America, the Middle East, and Africa have untapped opportunities for gene therapy CDMO providers to expand.
The growth of the gene therapy CDMO market varies across regions due to factors such as increasing healthcare investments, rising chronic disease prevalence, supportive regulations, and expanding adoption of advanced gene therapies. Below is a summary of market share and trends by region:
1. North America
- Market Share: Holds a significant portion of the global market
- Key Drivers: The region leads due to advanced healthcare infrastructure, strong biotech presence, significant investment in research, and early adoption of innovative gene therapies.
- Trends: Biotech startups and large CDMOs collaborating to expedite the commercialization of CAR-T and other gene therapies.
2. Europe
- Market Share: Substantial market share
- Key Drivers: Supportive regulatory frameworks, increasing clinical trials, and rising demand for personalized and advanced gene-based treatments.
- Trends: Use of automated and modular manufacturing platforms to enhance scalability and minimize costs of producing gene therapies.
3. Asia Pacific
- Market Share: Fastest-growing region with an annually increasing market share
- Key Drivers: Growing healthcare expenditure, rising chronic disease prevalence, government initiatives, and increasing adoption of advanced gene therapies.
- Trends: Increased government support for establishing gene therapy research facilities and promoting CDMO growth in the region.
4. South and Central America
- Market Share: Steadily growing market share
- Key Drivers: Expanding healthcare access, rising disease burden, and increasing interest in innovative therapeutics.
- Trends: Enhancement of contract manufacturing partnerships to increase local access to innovative products.
5. Middle East and Africa
- Market Share: Small market share, growing at a rapid pace
- Key Drivers: Improving healthcare infrastructure, government support for biotech, and rising awareness of advanced treatment options.
- Trends: The rise of specialized biomanufacturing centers and the use of public-private partnerships to advance gene therapy development.
Gene Therapy CDMO Market Players Density: Understanding Its Impact on Business Dynamics
High Market Density and Competition
Competition is strong due to the presence of established players such as Lonza Group AG and WuXi. Regional and niche providers add to the competitive landscape across regions.
The high level of competition urges companies to stand out by offering:
- Advanced products and services
- Compliance with regulatory guidelines
Opportunities and Strategic Moves
- Rising demand for gene therapies provides CDMOs with opportunities to expand capacity, enter new regions, and support commercialization.
- Companies pursue mergers, collaborations, and technological investments to enhance capabilities, streamline production, and strengthen their position in the growing gene therapy sector.
Other companies analyzed during the course of research:
- OmniaBio
- Rentschler Biopharma SE
- Recipharm AB
- Pfizer CentreOne
- Almac Group
- STEMCELL Technologies
- Eurofins
- Avid Bioservices
- Curia
- Excellos
- uBriGene Biosciences
- AGC Biologics Inc.
- Samsung Biologics Co., Ltd.
- Cytiva.
Gene Therapy CDMO Market News and Recent Developments
- In June 2025, Thermo Fisher Scientific announced the opening of a new facility in the Netherlands, specializing in ambient-to-cryogenic storage, clinical and commercial packaging, labeling and distribution, and clinical QP release services. The new current good manufacturing practice (cGMP) facility in Bleiswijk, Netherlands, provides pharma and biopharma customers with tailored, end-to-end support throughout the clinical supply chain for high-value therapies, including cell and gene therapies, biologics, antibodies, and vaccines.
- In June 2024, Charles River Laboratories International, Inc. and Captain T Cell announced an agreement for a plasmid DNA and retrovirus vector production program. As part of Charles River’s Cell and Gene Therapy (CGT) Accelerator Program (CAP), Captain T Cell will have access to established plasmid and viral vector CDMO capabilities, as well as advisory services, ahead of its plan to manufacture a therapy for solid tumor patients for a Phase I clinical trial.
Gene Therapy CDMO Market Report Coverage and Deliverables
The "Gene Therapy CDMO Market Size and Forecast (2026–2034)" report provides a detailed analysis of the market covering below areas:
- Gene therapy CDMO market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Gene therapy CDMO market trends, as well as market dynamics such as drivers, restraints, and opportunities
- Detailed PEST and SWOT analysis
- Gene therapy CDMO market analysis covering key trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the gene therapy CDMO market
- Detailed company profiles
Frequently Asked Questions
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For