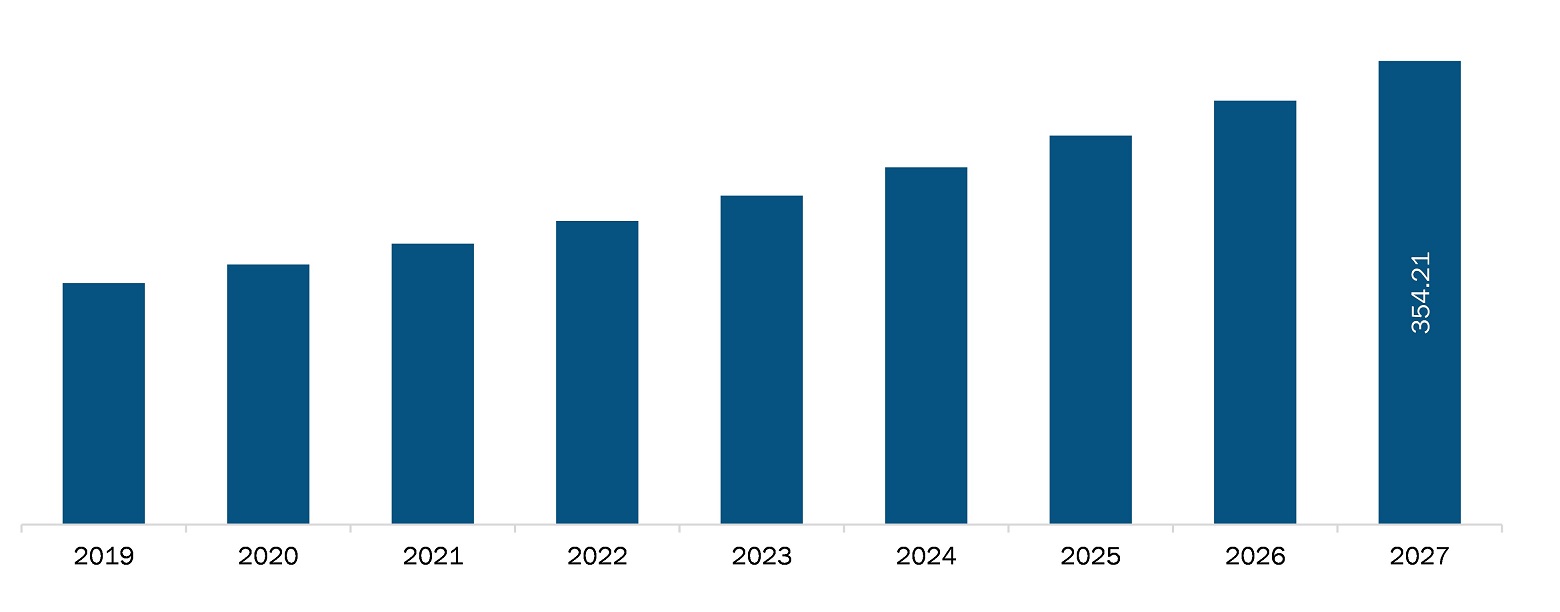
The North America microcatheters market is expected to reach US$ 354.21 million by 2027 from US$ 184.49 million in 2019; it is estimated to grow at a CAGR of 8.6% from 2020 to 2027.
The market growth is attributed to significantly increasing prevalence of chronic disorders, such as cardiovascular and neurovascular diseases, and rising elderly population across North America. However, product recall and stringent regulatory requirement, and lack of expert professionals hinder the market growth.
Micro-catheters are delivery devices used in minimally invasive applications. These small catheters are ideal for navigating the vast network of tiny veins found within the body. According to the World Health Organization (WHO), cardiovascular diseases (CVDs) are the most prominent cause of morbidity and mortality across the world. The CVDs include cerebrovascular disease, coronary heart disease, rheumatic heart disease, and so on. As per the American Heart Association (AHA) 2019 statistics, 121.5 million adults in the US (around 50% of the US adult population) suffer from a CVD. Therefore, the significantly rising incidence of CVDs across North America is likely to propel the demand for devices, such as microcatheters, that are required to treat these diseases. In addition, in the recent years, the prevalence of neurological disorders such as cerebral aneurysm has increased significantly across North America, which fuelled the demand for microcatheters used in their treatment procedures. Also, the rising geriatric population leads to rise in cardiovascular disease and neurological disease, which is contributing to the growth of the North America microcatheters market.
North America has been recording growing number of COVID-19 confirmed cases since its outbreak. The outbreak has adversely affected the adoption of and demand for neurovascular devices in neurosurgeries/elective surgeries. Several measures are being taken to prevent the spread of the novel coronavirus that causes COVID-19. Also, the rapidly rising number of COVID-19 confirmed cases have resulted in cancellation of doctor's appointment and decreased demand for the elective neurosurgery. In addition, there is a disruption in supply chain due to long period of lockdown. Moreover, the financial crisis among the masses in North America hampers the growth of the microcatheters market in the region.
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
NORTH AMERICA MICROCATHETERS MARKET SEGMENTATION
By Indication
- Neurovascular
- Coronary
- General Peripheral Vascular
By End User
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics
By Country
- US
- Canada
- Mexico
Company Profiles
- Boston Scientific Corporation
- Medtronic
- Merit Medical Systems Inc
- Terumo Corporation
- Biomerics LLC
- Asahi Intecc USA, Inc.
- Penumbra, Inc
- Stryker Corporation
- Teleflex Incorporated
North America Microcatheters Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2019 | US$ 184.49 Million |
| Market Size by 2027 | US$ 354.21 Million |
| CAGR (2020 - 2027) | 8.6% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Indication
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For