Urea Breath Segment and Laboratory Test Segment to Account for Largest Share in Helicobacter Pylori (H. pylori) Noninvasive Testing Market During 2022–2028
According to our latest study on Helicobacter Pylori (H. pylori ) Noninvasive Testing Market Forecast to 2028 – COVID-19 Impact and Global Analysis – by Test Type, Test Method, and End User," the market is expected to grow from US$ 596.82 million in 2021 to US$ 800.04 million by 2028; it is expected to grow at a CAGR of 4.4% from 2022 to 2028. The report highlights the key factors driving the market growth and prominent players with their developments in the market.
Based on test type, the Helicobacter Pylori (H. pylori ) Noninvasive Testing Market is segmented into serology, stool antigen, and urea breath. In 2021, the urea breath segment held the largest share of the market. In addition, owing to growing availability, the urea breath is expected to register the highest CAGR and will hold a major market share during the forecast period. Based on test method, the Helicobacter Pylori (H. pylori ) Noninvasive Testing Market is bifurcated into laboratory based and point-of-care. The laboratory based segment held a larger market share in 2021 and is likely to continue its dominance during the forecast period. Based on end user, the Helicobacter Pylori (H. pylori ) Noninvasive Testing Market is segmented into clinics, hospitals, diagnostic laboratories, and hospitals. The diagnostic laboratories segment held the largest market share in 2021 and will likely continue its dominance during the forecast period.
The global Helicobacter Pylori (H. pylori) non-invasive testing market is expected to grow significantly during the forecast period. The growth is mainly attributed to the global increase in the prevalence of H. pylori infection and the rapid use of non-invasive tests. Patients and healthcare providers have an increased preference for non-invasive tests due to their quick turnaround time for test results, increased patient compliance, and diminished discomfort. Moreover, rapid product launches & FDA approvals, as well as rising awareness campaigns by governments of various countries for the diagnosis of H. pylori, are positively impacting the market's growth. However, a low diagnosis rate due to low socioeconomic status or a low level of education of H. pylori is expected to hamper the Helicobacter Pylori (H. pylori) Noninvasive Testing Market growth.
The COVID-19 pandemic substantially influenced the global Helicobacter Pylori (H. pylori) non-invasive testing market. This is mainly due to the presence of H. pylori in COVID-19 patients causing abdominal pain and diarrhea. SARS-CoV-2 enters cells through binding angiotensin-converting enzymes (ACE-2) receptors, and H. pylori are responsible for increasing the expression of ACE-2 receptors in the gastrointestinal tract. Therefore, with substantial relation between H. pylori and COVID-19, there has been considerable demand for H. pylori non-invasive testing, positively impacting the market revenue growth during the forecast period.DiaSorin S.p.A.; Meridian Bioscience, Inc.; Exalenz Bioscience Ltd.; Alere; Thermo Fisher Scientific; Biomerica, Inc.; Abbott; and CerTest Biotec are among the leading companies operating in the global Helicobacter Pylori (H. pylori) non-invasive testing market.
Global Helicobacter Pylori (H. Pylori) Non-Invasive Testing Market, by Region, 2021 (%)
Helicobacter Pylori (H. pylori) Non-Invasive Testing Market Forecast to 2028 - Analysis By Test Type (Serology, Stool Antigen, and Urea Breath), Test Method (Laboratory Based and Point-of-Care), and End User (Hospitals, Clinics, and Diagnostic Laboratories)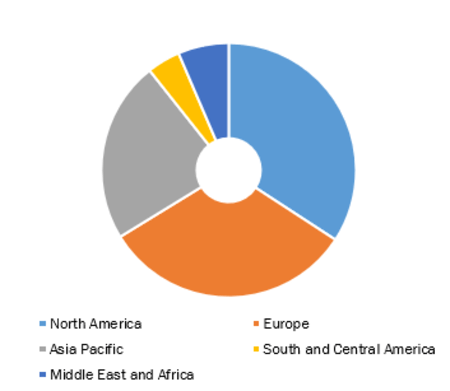
Helicobacter Pylori (H. pylori) Non-Invasive Testing Market 2028
Download Free Sample
Various organic and inorganic strategies are adopted by companies operating in the global H. pylori non-invasive testing diagnostics market. Organic strategies mainly include product launches and product approvals. Further, acquisitions, collaborations, and partnerships are among the inorganic growth strategies witnessed in the market. These growth strategies allow the market players to expand their businesses and enhance their geographic presence, thereby contributing to the overall market growth. Further, acquisition and partnership strategies help the market players strengthen their customer base and expand their product portfolios. A few of the significant developments by key players operating in the global H. pylori non-invasive testing diagnostics market are listed below.
- In 2021, Biomerica signed a five-year distribution agreement with a Canadian partner to distribute and market Biomerica's new and proprietary H. pylori test, called hp+detect. The product has been developed to identify and monitor H. pylori infection. Biomerica's distribution partner will now seek Health Canada clearance using the clinical data generated by Biomerica using hp+detect to sell the product in Canada.
- In 2020, Meridian Bioscience entered a definitive agreement to acquire Exalenz for ~US$ 49 million. Under the terms of the agreement, Meridian will be adding Exalenz's flagship BreathID® Breath Test Systems, a urea breath platform for the detection of H. pylori.
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com