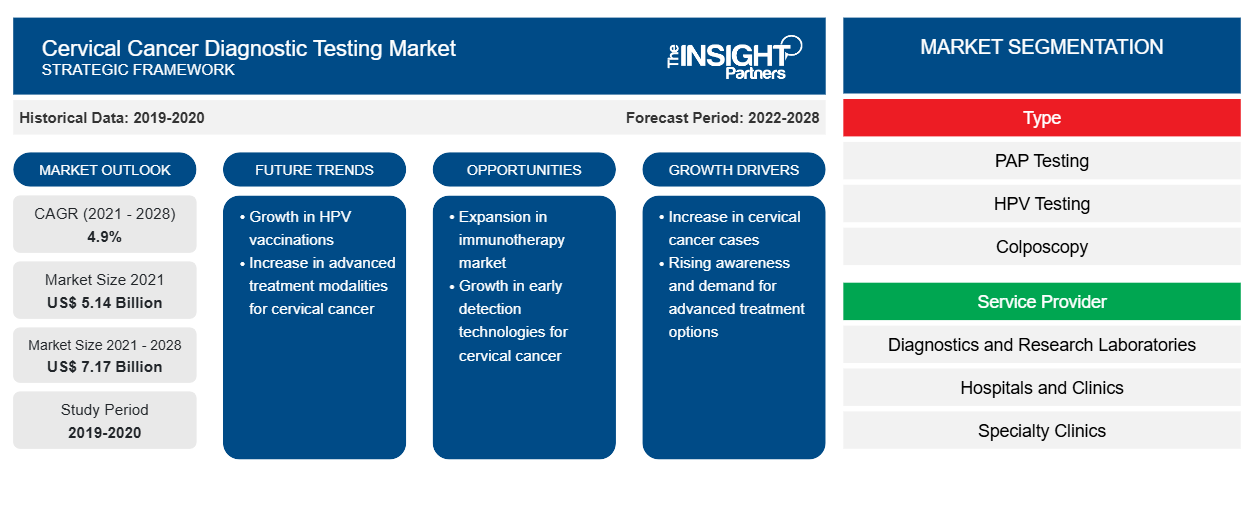
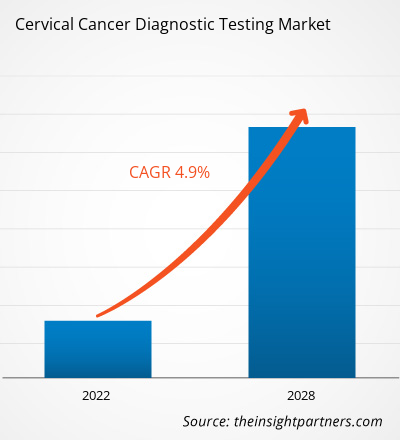
The cervical cancer diagnostic testing market is projected to reach US$ 7,165.92 million by 2028 from US$ 5,141.25 million in 2021; it is expected to grow at a CAGR of 4.9% from 2021 to 2028.
Cervical cancer has a significant mortality rate (almost 50%), which can be lowered with early detection and prevention. Precancerous alterations offer opportunities for prevention and treatment because disease progression is often delayed. However, due to a lack of awareness or access to diagnostic services, many cases are detected at later stages of illness progression. Human Papillomavirus (HPV) is a primary cause of cervical cancer that is typically transmitted through sexual contact. There are over 100 HPV strains, and 13 of which are high-risk or carcinogenic. The active gene - E6 or E7 - determines whether an HPV strain is high or low risk. E6 binds to p53, causing proteolytic destruction. E7, on the other hand, binds to retinoblastoma; the binding displaces previously attached transcription factors, resulting in the cell cycle being stopped and apoptosis regulation being inhibited. The HPV test detects human papillomavirus in cervical cells. Other screening approaches employed in low-resource areas of developing countries are HPV DNA testing and visual inspection. Cervical screening on a regular basis aids in an early detection of cervical cancer, lowering the mortality rate from the disease. Every three years, all women between the ages of 21 and 65 are advised to have a Pap test, and women between the ages of 30 and 65 are advised to have both a Pap and an HPV test. In terms of revenue, this type dominated the cervical cancer screening market in 2021, and it is expected to continue its dominance during the forecast period.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Cervical Cancer Diagnostic Testing Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Increasing Prevalence of Cancer to Drive Cervical Cancer Diagnostic Testing Market Growth During Forecast Period
Cancer has emerged as a leading cause of death across the world. According to the World Health Organization (WHO), cancer was the first leading cause of death in the people of below 70 years in 183 countries and fourth leading cause of death in 123 countries worldwide in 2019. In addition, according to data published by the WHO in March 2021, ~10 million deaths occurred in 2020 due to different cancer types. The rising incidence of cervical cancer among women worldwide drives the cervical cancer diagnostic testing market growth. According to the WHO, more than 270,000 deaths of women are recorded due to cervical cancer each year. Cervical cancer mortality rates are greater in low-income countries due to the late diagnosis of the disease. The market is predicted to grow in the coming years due to the increasing acceptance of cervical cancer diagnostic tests for the early detection of the disease.
The increasing prevalence of cancer has created burden on the healthcare systems across the world. According to International Agency for Research on Cancer (IARC), the global burden of new cancer cases is estimated to reach ~ 27.5 million by 2040, and the disease is likely to be a cause of ~163 million deaths by that year. Factors such as lifestyle changes, smoking, reduced physical activities, and uncertain health and climatic conditions are would lead to even greater burden of cancer in the world in the coming years.
Type-Based Insights
Based on type, the cervical cancer diagnostic testing market is segmented into PAP testing, HPV testing, colposcopy, cervical biopsies, cystoscopy, and others. In 2021, the PAP testing segment held the largest share of the market, and it is estimated to register the highest CAGR during 2021–2028.
Service Provider-Based Insights
Based on the service provider, the market is segmented into research, diagnostics and research laboratories, hospitals and clinics, specialty clinics, and home care services. In 2021, the diagnostics and research laboratories segment held the largest share of the market and is expected to register the highest CAGR in the market during 2021–2028.
Companies operating in the cervical cancer diagnostic testing market are adopting various strategies such as, product innovations, to meet the evolving customer demands across the world and maintain their brand name in the global market.
Cervical Cancer Diagnostic Testing Market – Segmentation
The cervical cancer diagnostic testing market is segmented on the basis of type, and service provider. Based on type, the market is sub-segmented into PAP testing, HPV testing, colposcopy, cervical biopsies, cystoscopy, and others. Based on the service provider, the market is further segmented into diagnostics and research laboratories, hospitals and clinics, specialty clinics, and home care services.
In terms of geography, the cervical cancer diagnostic testingmarket is segmented into North America (the US, Canada, and Mexico), Europe (France, Germany, Italy, the UK, Spain, and Rest of Europe), Asia Pacific (Australia, China, India, Japan, South Korea, and Rest of APAC), the Middle East & Africa (Saudi Arabia, South Africa, the UAE, and Rest of MEA), and South and Central America (Brazil, Argentina, and Rest of SCAM).
Cervical Cancer Diagnostic Testing Market Regional Insights
The regional trends and factors influencing the Cervical Cancer Diagnostic Testing Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Cervical Cancer Diagnostic Testing Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Cervical Cancer Diagnostic Testing Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 5.14 Billion |
| Market Size by 2028 | US$ 7.17 Billion |
| Global CAGR (2021 - 2028) | 4.9% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Cervical Cancer Diagnostic Testing Market Players Density: Understanding Its Impact on Business Dynamics
The Cervical Cancer Diagnostic Testing Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Cervical Cancer Diagnostic Testing Market top key players overview
Company Profiles
- F. Hoffmann-La Roche Ltd.
- Abbott
- Quest Diagnostics Incorporated
- QIAGEN
- Hologic, Inc.
- DYSIS Medical Inc.
- Femasys Inc.
- Guided Therapeutics, Inc.
- Cooper Companies, Inc.
- BD
Frequently Asked Questions
Which region is expected to witness significant demand for the cervical cancer diagnostic testing market in the coming years?
Which is the most influencing segment growing in the cervical cancer diagnostic testing market report?
What is the market value of the cervical cancer diagnostic testing market based on region?
Who are the major players in the cervical cancer diagnostic testing market?
What is Cervical Cancer Diagnostic Testing?
What are the factors impacting the cervical cancer diagnostic testing market?
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Related Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





















 Get Free Sample For
Get Free Sample For