Análisis de la cuota de mercado y oportunidades de validación y verificación de dispositivos médicos 2025-2031
Medical Device Validation and Verification Market Report Analysis
Medical Device Validation and Verification Market
-
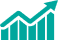
CAGR (2025 - 2031)11.1% -
Market Size 2024
US$ XX million -
Market Size 2031
US$ XX Million

Report Coverage
- Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Key future trends
- Detailed PEST/Porter’s Five Forces and SWOT analysis
- Industry landscape and competition analysis & recent developments
- Detailed company profiles
- Global and regional market analysis covering key market trends, major players, regulations, and recent market developments
Key Players
- SGS SA
- QuEST Global Services Pte. Ltd
- Intertek
- Element Materials Technology
- North American Science Associates, Inc
- Eurofins Scientific SE
- Charles River
- Sterling Medical Devices
- Pacific Biolabs
Regional Overview
- Norteamérica
- Europa
- Asia-Pacífico
- América del Sur y Central
- Oriente Medio y África
Market Segmentation
 By Área terapéutica
By Área terapéutica
- Nefrología
- Respiratoria
- Neurología
- Oncología
- Otorrinolaringología
- Cardiovascular
- Dermatología
 By Aplicación
By Aplicación
- Diagnóstico
- Terapéutica
- Implantes
 By Tecnología
By Tecnología
- Pruebas mecánicas
- biológicas
- pruebas de seguridad eléctrica
 By Geografía
By Geografía
- América del Norte
- Europa
- Asia-Pacífico
- América del Sur y Central
- Oriente Medio y África
 Get Free Sample For
Get Free Sample For