Drug Development and Manufacturing Segment Drives Cell Therapy CDMO Market Growth
According to our new research study named "Cell Therapy CDMO Market Forecast to 2031 – Global Analysis – by Service Type, End User, and Geography," the market was valued at US$ 4.12 billion in 2024 and is projected to reach US$ 21.92 billion by 2031; it is expected to register a CAGR of 27.1% during 2025–2031. The increasing prevalence of chronic and rare diseases, surging outsourcing of cell therapy manufacturing by biotech and pharma companies, and expanding clinical trials for innovative therapies drive the adoption of cell therapy CDMO. The rising integration of AI and digital transformation, and increased adoption of advanced and automated manufacturing technologies, are projected to bring new cell therapy CDMO market trends in the coming years.
CDMOs develop, manufacture, and test cell-based therapies for biotech companies, enabling scalable production, quality control, and regulatory compliance. The rising cell therapy approvals and an increasing number of clinical pipelines are driving the global cell therapy CDMO market growth.
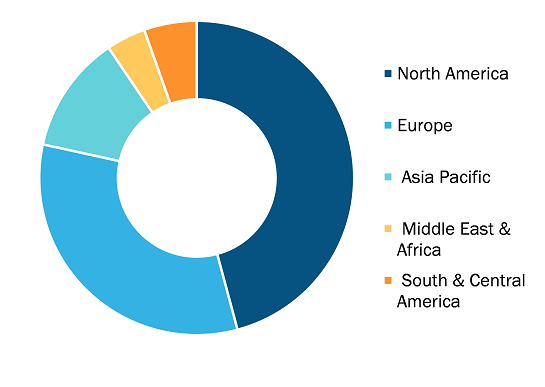
Cell Therapy CDMO Market, by Region, 2024 (%)
Cell Therapy CDMO Market Size & Growth Opportunities by 2031
Download Free SampleCell Therapy CDMO Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Service Type (Drug Development and Manufacturing, Testing and Regulatory Services, and Other Service Types), End User (Pharmaceutical Companies, Biopharmaceutical Companies, and Other End Users), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)
Source: The Insight Partners Analysis
Biopharma companies outsourcing their operations is a major factor in the volume increase, thus contributing to the rising cell therapy CDMO market size. The complexity of the production process and the requirement of expertise in regulatory matters propel the demand for CDMOs. Besides, the growth is well supported by the improvements in automation and the widespread use of oncology indications. There is intense demand for a production infrastructure that is cost-efficient and scalable, favoring the demand of CDMOs.
Cell Therapy CDMO Market Analysis Based on Segmental Evaluation:
Based on service type, the cell therapy CDMO market is segmented into drug development and manufacturing, testing and regulatory services, and other service types. The drug development and manufacturing segment held a significant cell therapy CDMO market share in 2024. The wide variety of biotech startups and established companies lacks the proper infrastructure and know-how. CDMO has the expertise for this by offering full support from production scale-up to regulatory compliance and ensuring the production is efficient. Technological advancements, including AI integration for optimizing manufacturing workflows and continuous processing systems, have reduced the time to reach the market. Additionally, the increasing number of clinical trials—over 1,000 trials reported by the Alliance for Regenerative Medicine as of 2024—has created demand for facilities with large-scale capabilities, and hence, the continuous processing systems have made it easier for companies to keep up with the burgeoning market.
The FDA's and EMA's expedited pathways are among the favorable regulatory frameworks that push firms to invest in the development of new therapies. In developing countries, where chronic and rare diseases are predominant, and hence the demand for pharmaceutical companies to carry out R&D and introduce new treatments is high, driving the outsourcing of services. AGC Biologics' 2020 partnership with Autolus Therapeutics for viral vector supply in the obe-cel CAR-T therapy enabled the company to penetrate the market. Bristol-Myers Squibb's 2023 agreement with a CDMO for Breyanzi production involved the modification and expansion of each patient’s cells.
The geographical scope of the cell therapy CDMO market report encompasses an assessment of the market performance in North America, Europe, the Asia Pacific, South America, Central America, the Middle East, and Africa. North America dominated the cell therapy CDMO market share in 2024. There is a growing need for sophisticated cell-based therapies, such as CAR-T cell therapy and stem cell therapy, because of their immense potential in treating and dealing with cancers, autoimmune diseases, as well as rare genetic disorders. Investment by private and public sectors in research related to regenerative medicine drives the need for cell therapy-based manufacturing services provided by CDMOs. North America, especially the US, enjoys favorable regulatory settings, thanks to guidance by regulatory authorities such as the FDA, making way for faster processing of cell therapeutic products.
The increased rate of chronic diseases, accompanied by a rising number of senior citizens, is driving up the need for innovative medical treatments. Third-party cell therapy CDMOS, life-science companies, especially biotech’s, get a chance to overcome issues of complicated processing, as well as high fabrication costs, enabling these companies to devote their complete energy toward research and further refinement of clinical breakthroughs, thereby boosting the cell therapy CDMO market growth.
Advances in technological processing of cell fabrication, such as automated processing, Cryopreservation, and Quality Control, make way for increased safety as well as efficiency of cell therapeutic manufacturing. Hence, the above-mentioned factors collectively support the growth of the global cell therapy CDMO market.
WuXi Biologics Inc., Charles River Laboratories International Inc., Catalent Inc., Lonza Group AG, National Resilience Inc., Thermo Fisher Scientific Inc., AGC Biologics AS, Takara Bio Inc., FUJIFILM Holdings Corp, and SK Pharmteco Inc. are among the leading companies profiled in the cell therapy CDMO market report.
Based on service type, the cell therapy CDMO market is segmented into drug development and manufacturing, testing and regulatory services, and other service types. By end user, the market is classified into pharmaceutical companies, biopharmaceutical companies, and other end users. Geographically, the cell therapy CDMO market is segmented into North America (US, Canada, and Mexico), Europe (France, Germany, UK, Spain, Italy, and the Rest of Europe), Asia Pacific (China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific), the Middle East and Africa (Saudi Arabia, South Africa, the UAE, and the Rest of Middle East and Africa), and South and Central America (Brazil, Argentina, and the Rest of South and Central America).
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com