Rising Incidence of Gastrointestinal Conditions and Increasing Technological Advancements Drive Endotherapy Devices Market Growth
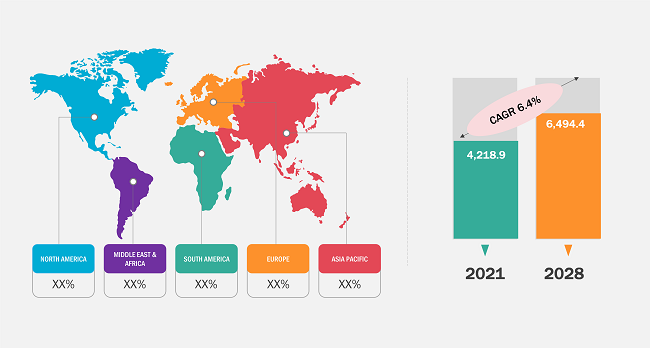
According to our latest study on "Endotherapy Devices Market Forecast to 2028 – COVID-19 Impact and Global Analysis – by Product, Application, End User, and Geography," the market was valued at US$ 4,218.9 million in 2021 and is likely to reach US$ 6,494.4 million by 2028; it is expected to grow at a CAGR of 6.4% from 2022 to 2028.
Endotherapy Devices Market Share, Trends & Growth by 2031
Download Free SampleEndotherapy Devices Market Size and Forecasts (2021 - 2031), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: by Product (Gastrointestinal Devices and Accessories, Endoscopic Retrograde Cholangio Pancreatography Devices and Accessories, and Others), Application (Bronchoscopy, Arthroscopy, Laparoscopy, Urology Endoscopy, Neuroendoscopy, Gastrointestinal Endoscopy, and Other), End User (Hospitals, Clinics, and Others), and Geography (North America, Europe, Asia Pacific, and South and Central America)
The rising incidence of gastrointestinal diseases, including constipation, IBS, hemorrhoids, and internal hemorrhoids, and increasing geriatric population are boosting the endotherapy devices market growth. In the US, irritable bowel syndrome (IBS) is common and affects about 5% of the population (about 1 in 20 people). Furthermore, in the country, constipation is the most common gastrointestinal complaint affecting 4 million people and resulting in 2.5 million doctor visits annually. Additionally, increasing preference for minimally invasive procedures and technological advancements are expected to create lucrative opportunities for the market in the coming years. However, the high cost of endotherapy devices and the lack of skilled technicians hamper the market growth.
According to the GI Alliance, digestive disease is excepted to rise among Americans in coming years. Twenty million Americans suffer from chronic digestive diseases, out of which 25% of surgical operations are performed. Moreover, in the US, digestive diseases rank third among illnesses in total economic cost. Fourteen million acute digestive diseases are treated each year, including one-third of all malignancies and some common acute infections. As per the United European Gastroenterology (UEG), in Europe, across all ages, around one million deaths occur each year due to gastrointestinal (GI) and liver disorders and are associated with substantial morbidity and healthcare costs. Moreover, owing to the rising geriatric population and growing incidence and prevalence of many GI disorders across the region, the gastrointestinal disease burden is excepted to inevitably increase.
Due to growing cases of digestive disease in Europe, organizations are expanding their portfolio across different regions. In May 2018, Spot Ex Endoscopic Tattoo received CE Mark and is now available in Europe. It is used to endoscopically mark cancerous tissue, suspicious lesions, and polyps for easier future identification at follow-up colonoscopy or surgery. Additionally, on September 202, Blueprint Medicines announced European Commission Approval of AYVAKYT (avapritinib) for the treatment of adults with unresectable or metastatic PDGFRA D842V mutant gastrointestinal stromal tumors. It is the first highly effective treatment option approved in Europe for PDGFRA D842V mutant GIST
Technologically advanced endotherapy devices offer several benefits, such as safety, enhanced ergonomics, and increased efficiency, which boosts their significance and use at healthcare facilities. In December 2021, Olympus Corporation released single-use Foreign Body Retrieval devices product line. The company supplies a broad spectrum of specialized single-use grasping forceps to help rescue foreign bodies, stents, and excised tissue. These single-use products are expected to replace reusable devices. Additionally, in October 2021, Fujifilm launched El-740D/S, the dual-channel endoscope cleared by the US FDA. The endoscope can be used in both upper and lower gastrointestinal applications. It has the capability to provide exceptional imaging quality and features dual channel diameters of 3.7 mm and 3.2 mm. It also aids visualization for diagnostic and therapeutic procedures. Thus, the high demand and availability of innovative devices for better diagnosis and treatment are expected to bolster the endotherapy devices market in the coming years.
Boston Scientific Corporation, CONMED Corporation, FUJIFILM Holdings Corporation, HOYA Corporation, Johnson & Johnson, KARL STORZ GmbH & Co KG, Medtronic Plc., Olympus Corporation, Stryker Corporation, and Smith & Nephew Plc. are among the key players profiled during the study of the endotherapy devices market. Several other major companies were studied and analyzed during this research study to get a holistic view of the market and its ecosystem.
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com