Drug Development and Manufacturing Segment Drives Gene Therapy CDMO Market Growth
According to our new research study named "Gene Therapy CDMO Market Forecast to 2034 – Global Analysis – by Service Type, End User, and Geography," the market was valued at US$ 2.60 billion in 2025 and is projected to reach US$ 14.17 billion by 2034; it is expected to register a CAGR of 20.3% during 2026–2034. The increasing prevalence of chronic and genetic diseases, surging regulatory approvals and commercialization, and growing popularity of outsourcing gene therapy manufacturing by companies drive the adoption of gene therapy CDMO. Advanced automation, digitalization, and the growing demand for personalized and rare disease therapies are projected to drive new gene therapy CDMO market trends in the coming years.
Gene therapy CDMOs are specialized companies providing development and manufacturing services for gene therapies, supporting vector production, process optimization, quality control, regulatory compliance, and scalable commercialization for biopharmaceutical developers worldwide. The rising prevalence of genetic disorders, the expansion of clinical pipelines, and increasing demand for outsourcing are driving global gene therapy CDMO market growth. Additionally, the complexity of manufacturing, rapidly advancing technology, strong regulatory support, and the influx of funding are driving growth in the gene therapy CDMO market size. At the same time, pharmaceutical companies are seeking global partners that can deliver cost efficiency, scalability, and faster time-to-market while helping manage risks and ensure sustainable long-term growth are some more factors contributing to the rising gene therapy CDMO market size.
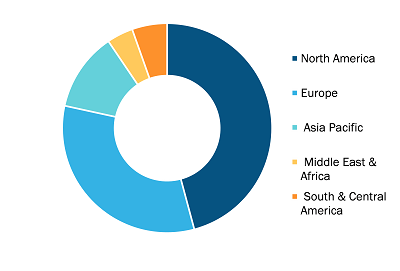
Gene Therapy CDMO Market, by Region, 2025 (%)
Gene Therapy CDMO Market Size, Share & Forecast to 2034
Download Free Sample
Gene Therapy CDMO Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Service Type (Drug Development and Manufacturing, Testing and Regulatory Services, and Other Service Types), End User (Pharmaceutical Companies, Biopharmaceutical Companies, and Other End Users), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)
Gene Therapy CDMO Market Size, Share & Forecast to 2034
Download Free SampleGene Therapy CDMO Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Service Type (Drug Development and Manufacturing, Testing and Regulatory Services, and Other Service Types), End User (Pharmaceutical Companies, Biopharmaceutical Companies, and Other End Users), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)
Source: The Insight Partners Analysis
Gene Therapy CDMO Market Analysis Based on Segmental Evaluation:
Based on service type, the gene therapy CDMO market is segmented into drug development and manufacturing, testing and regulatory services, and other service types. The drug development and manufacturing segment held a significant gene therapy CDMO market share in 2025. The segment is developing rapidly owing to factors such as pharmaceutical and biotechnology research, the advent of complex biologics, and the need for a faster time-to-market. In the process of outsourcing to specialized partners, companies achieve cost reductions, improved risk management, and access to advanced technologies. The demand is further fueled by regulatory requirements that are hard to meet, limited in-house manufacturing capacity, and increased activity in clinical trials. Moreover, the rise of personalized medicine, gene and cell therapies, and the global healthcare market has led to increased reliance on contract development and manufacturing organizations for scalable, compliant, and high-quality production solutions.
The geographical scope of the gene therapy CDMO market report encompasses an assessment of the market performance in North America, Europe, the Asia Pacific, South America, Central America, the Middle East, and Africa. North America dominated the gene therapy CDMO market share in 2025. One of the key factors driving gene therapy development and the resulting growth of the CDMO market in North America is the region’s strong biotechnology infrastructure, supported by extensive research and development funding. Regulatory measures prompt innovation and reduce time-to-market, leading companies to rely on CDMOs for compliance support and large-scale production, thereby boosting gene therapy CDMO market growth.
Another reason for outsourcing is that pharmaceutical companies often lack adequate in-house facilities for gene therapy manufacturing. As a result, they increasingly rely on external partners to access expertise in viral vector and cell processing, quality control, and GMP-compliant production that can be readily scaled. Furthermore, collaborations and investments in advanced manufacturing—including workforce training and state-of-the-art technologies such as automation and single-use systems—further strengthen the region’s CDMO ecosystem. Increased incidences of genetic diseases and cancer in the region have led to a demand for personalized medicine, which in turn supports the continuous growth of the gene therapy CDMO market in North America.
Moreover, the region's strong healthcare infrastructure, favorable regulatory policy, fast-paced technological development, and increasing investments in biomanufacturing are additional drivers of market growth. In October 2024, OmniaBio Inc. opened Canada’s largest biomanufacturing facility dedicated exclusively to cell and gene therapies in Hamilton, Ontario. The 120,000-square-foot facility received a US$ 580 million investment, enabling it to address challenges in product commercialization by offering a full range of services from start to finish.
In August 2025, COFEPRIS approved a collaboration between the local firm Liomont and US-based Andelyn Biosciences for technology transfer in plasmid engineering. In early 2026, the Mexican Biotechnology Association announced funding for GMP upgrades at Cinvestav’s bioprocessing labs, enhancing quality assurance protocols. Hence, the above-mentioned factors collectively support the growth of the global gene therapy CDMO market.
Charles River Laboratories International Inc., Catalent Inc., Lonza Group AG, WuXi Biologics Inc., National Resilience Inc., Thermo Fisher Scientific Inc., AGC Biologics AS, Takara Bio Inc., FUJIFILM Holdings Corp, and SK Pharmteco Inc. are among the leading companies profiled in the gene therapy CDMO market report.
Based on service type, the gene therapy CDMO market is segmented into drug development and manufacturing, testing and regulatory services, and other service types. By end user, the market is classified into pharmaceutical companies, biopharmaceutical companies, and other end users. Geographically, the gene therapy CDMO market is segmented into North America (the US, Canada, and Mexico), Europe (France, Germany, the UK, Spain, Italy, and the Rest of Europe), Asia Pacific (China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific), the Middle East and Africa (Saudi Arabia, South Africa, the UAE, and the Rest of Middle East and Africa), and South and Central America (Brazil, Argentina, and the Rest of South and Central America).
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com