Matrix Segment to Dominate Transdermal Medical Patch Market During 2023–2028
According to our new research study on “Transdermal Medical Patch Market –Global Analysis and Forecast – by Type, Application, and Distribution Channel,” the transdermal medical patch market size is expected to reach US$ 10,799.74 million by 2028 from US$ 8,247.21 million in 2022; it is estimated to record a CAGR of 4.0% from 2023 to 2028. The report highlights the trends prevalent in the global transdermal medical patch market, and the drivers and deterrents pertaining to its growth.
Based on type, the transdermal medical patch market is segmented into matrix, reservoir, single-layer drug-in-adhesive, multi-layer drug-in-adhesive, and vapour patch. The matrix segment held the largest market share in 2022. Furthermore, the same segment is anticipated to record a significant growth rate during the forecast period. The matrix patches segment is likely to register significant growth owing to ease of use. These patches can be utilized for various conditions, such as allergies, dermal conditions, and pain. Further, increasing research and development activities to validate the feasibility of transdermal patches for treating diseases is likely to boost the new product introduction, thus driving the transdermal medical patch market growth.
Global Transdermal medical patch Market, by Region, 2022 (%)
Transdermal Medical Patch Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Single Layer Drug-in-Adhesive, Multi-layer Drug-in-Adhesive, Reservoir, Vapor Patch, and Matrix), Application (Neurologic Conditions, Pain Management, Nicotine Cessation, Cardiovascular Disorders, Hormonal Therapy, and Others), and Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies)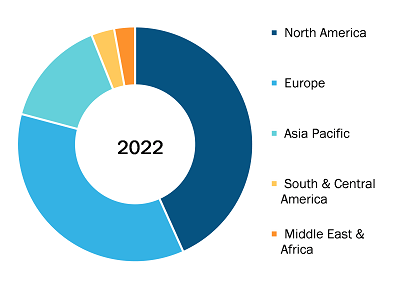
Transdermal Medical Patch Market Size & Emerging Trends 2034
Download Free Sample
Source: The Insight Partners Analysis
Impact of COVID-19 Pandemic on Transdermal Medical Patch Market.
The transdermal medical patch market had witnessed a shortfall at the beginning of the COVID-19 crisis owing to factors such as disruption in supply chain and demand due to lockdown announced by the majority of countries across the world that caused disruptions in manufacturing and supply chain operations. During the pandemic the healthcare industry focused primarily on addressing the immediate challenges related to the virus, such as testing, treatment, and vaccination efforts. Non-essential medical procedures and treatments were often postponed or scaled back to prioritize COVID-19 patients. This may have had an impact on the overall demand for medical patches, particularly for nonurgent applications. On the other hand, the pandemic also highlighted the importance of remote monitoring and telemedicine solution as healthcare systems sought to limit in-person visits and reduce the risk of transmission. Furthermore, the pandemic led to an increased focus on drug delivery systems, as researchers and pharmaceutical companies explored innovative ways to administer vaccines and treatment. Medical patches that can deliver drugs or vaccine transdermal offered advantages in terms of ease-of-use patient compliance, and potentially more stable drug formulation. Overall, the impact of COVID-19 on the medical patch market in across the globe is likely to be mix of challenges and opportunity. Factors such as the specific application of the patches, regulatory considerations, healthcare systems priorities, and technological advancement will play a role in determining the market dynamics during and after the pandemic which could lead to the transdermal medical patch market growth in the forecasted period. Hence, the outbreak of COVID-19 has positively impacted the transdermal medical patch market.
Hisamitsu Pharmaceutical Co Inc, Medline Industries LP, Johnson & Johnson, Novartis AG, Teva Pharmaceutical Industries Ltd, UCB SA, Viatris Inc, Endo Pharmaceuticals Inc, Boehringer Ingelheim International GmbH, and Corium, LLC are a few key companies operating in the transdermal medical patch market. These companies adopt product innovation strategies to meet evolving customer demands, which allows them to maintain their brand name.
A few of the recent developments in the global transdermal medical patch market are mentioned below:
- In September 2022, Corium, Inc launched ADLARITY (donepezil transdermal system) and is available for prescription in the U.S. for treating patients with mild, moderate, or severe dementia of Alzheimer's type.
- In June 2022, Hisamitsu Pharmaceutical Co., Inc. has been approved for the additional indications of “low back pain, humeroscapular periarthritis, cervico-omo-brachial syndrome and tenosynovitis” for ZICTHORU Tapes, transdermal, pain treatment NSAID patch in Japan. The product was approved for manufacturing and marketing approval for “analgesia in various cancer” in March 2021. This approval is based on the data of Phase III clinical studies that evaluated the efficacy and safety of the product in patients with low back pain, humeroscapular periarthritis, cervico-omo-brachial syndrome and tenosynovitis. With this approval of the additional indications, Hisamitsu expects the product to be a new option for the treatment of low back pain, humeroscapular periarthritis, cervico-omo-brachial syndrome and tenosynovitis.
- In August 2021, Hisamitsu Pharmaceutical Co., Inc. submitted a new drug application for the additional indications of “low back pain, humeroscapular periarthritis, cervico-omo-brachial syndrome and tenosynovitis” for ZICTHORU Tapes, transdermal, pain treatment NSAID patch in Japan. Hisamitsu has confirmed the efficacy and safety of the product in clinical trials in patients with low back pain and patients with humeroscapular periarthritis, cervico-omo-brachial syndrome, and tenosynovitis. The product is a systemic transdermal formulation developed using Hisamitsu’s TDDS (Transdermal Drug Delivery System) technology.
- In August 2021, Hisamitsu Pharmaceutical Co., Inc. announced the approval of the additional indications of cancer pain relief for pediatric patients for FENTOS Tapes, a transdermal pain management patch in Japan. Hisamitsu Pharmaceutical conducted a clinical study in pediatric cancer pain patients (aged 2 to 19 years old) and confirmed the efficacy and safety of the product. With this approval, the product became the first strong opioid patch in Japan with an indication for pediatric cancer pain. Hisamitsu Pharmaceutical expects the product to be a new option for treating cancer pain.
The report segments the global transdermal medical patch market as follows:
Based on type, the transdermal medical patch market is segmented into matrix, reservoir, single-layer drug-in-adhesive, multi-layer drug-in-adhesive, and vapour patch. Based on application, the transdermal medical patch market is segmented into pain management, neurologic conditions, nicotine cessation, cardiovascular disorders, hormonal therapy, and others. By distribution channel, the transdermal medical patch market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. By geography, the transdermal medical patch market is segmented into North America (the US, Canada, and Mexico), Europe (France, Germany, the UK, Spain, Italy, and the Rest of Europe), Asia Pacific (China, India, Japan, Australia, South Korea, and the Rest of APAC), the Middle East & Africa (Saudi Arabia, the UAE, South Africa, and the Rest of MEA), and South & Central America (Brazil, Argentina, and the Rest of South & Central America).
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com