Page Updated:
Jul 2021
The micro catheters and micro guidewires market in APAC is expected to grow from US$ 279.91 million in 2021 to US$ 422.26 million by 2028; it is estimated to grow at a CAGR of 6.0% from 2021 to 2028.
The China, Japan, and India are major economies in APAC. Growing number of product approvals and launches is the major factor driving the growth of APAC micro catheters and micro guidewires market. Extended functionalities and technologies incorporated in new devices are contributing to the rise in demand for micro catheters and micro guidewires. In March 2021, Transit Scientific received CE approval for its XO CrossO Microcatheter platform. The new microcatheter incorporates novel non-tapered design, which offers better functionality. Moreover, the new platform delivers better torque response, trackability, and new pushability levels during a surgical procedure. Further, Also, in May 2019, Embolx, Inc. introduced Sniper K-tip microcatheter under sniper balloon occlusion microcatheter. This newly introduced microcatheter is especially designed for an arterial embolization procedure. Furthermore, in August 2020, Scientia Vascular received FDA approval for its Zoom Wire 14 guidewire. The new device incorporates capabilities to gain access of nervous system during hemorrhagic and ischemic stroke and other associated vascular procedures. Thus, the growing number of product launches and approvals are estimated to offer lucrative opportunities for the growth of the micro catheter and micro guidewires market during the forecast period, which is further anticipated to drive the market in APAC.Countries in Asia Pacific are facing challenges due to increasing incidences of COVID-19. The outbreak of the COVID-19 pandemic has come from China. The spread of infection has affected various Asian countries such as India, South Korea, Australia, Malaysia, Singapore, and others. Countries in Asia Pacific are facing challenges due to increasing incidences of COVID-19. Currently, India is the second worse hit country worldwide. Many measures have been implemented to contain the spread of COVID-19, which have resulted in significant operational disruption for many companies including in the healthcare industry. Staff quarantine, supply-chain failures, and reductions in demand have generated serious complications for companies.The outbreak has severely affected the tourism industry and imposed supply chain disruptions; moreover, low-income countries face additional challenges due to the shortage of healthcare infrastructure, restrictions imposed by hospitals to the suppliers, decreasing in demand of neurovascular devices and others. Restrictive measures have been put forth in South Korea, Malaysia, Singapore, the Philippines, and India to prevent the disease transmission. These restrictive measures are anticipated to impact the supply chain and the availability of medical devices in these countries. It has affected disposable incomes and has also resulted in unemployment. Furthermore, business strategies such as acquisitions, mergers, and partnerships are adversely affected by the imposition of complete lockdown. Such obstructions in business activities are projected to hamper the adoption of various technologically advanced healthcare systems, including microcatheters and micro guidewires 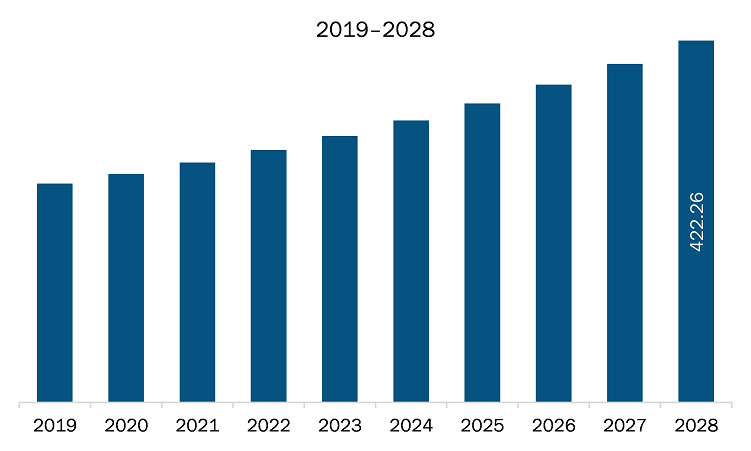
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
APAC Micro Catheters and Micro Guidewires Market Segmentation
APAC Micro Catheters and Micro Guidewires Market – By Product Type
- Micro Catheter
- Micro Guidewire
APAC Micro Catheters and Micro Guidewires Market – By Indications
- Cardiology
- Neurology
- Oncology
- Intravascular Embolization
- Transcatheter Arterial Chemo Embolization
- Urology
- Otolaryngology
- Others
APAC Micro Catheters and Micro Guidewires Market – By End User
- Hospitals
- Ambulatory Surgical Centres
- Specialty Clinics
APAC Micro Catheters and Micro Guidewires Market – By Country
- China
- Japan
- India
- South Korea
- Australia
- Rest of APAC
APAC Micro Catheters and Micro Guidewires Market -Companies Mentioned
- B. Braun Melsungen AG
- BD
- Boston Scientific Corporation
- Cardinal Health Inc
- Cook Medical LLC
- Medtronic
- Stryker Corporation
- Teleflex Incorporated
- Terumo Corporation
- Tokai Medical Products, Inc
Asia Pacific Micro Catheters and Micro Guidewires Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 279.91 Million |
| Market Size by 2028 | US$ 422.26 Million |
| CAGR (2021 - 2028) | 6.0% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
Asia-Pacific
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends
Our Clients






























Sales Assistance
US: +1-646-491-9876
UK: +44-20-8125-4005
Email:
sales@theinsightpartners.com
Chat with us

87-673-9708

ISO 9001:2015





 Get Free Sample For
Get Free Sample For