Circulating Tumor Cell (CTC) Diagnostics Market Trends and Future Insights (2026-2034)
Circulating Tumor Cell (CTC) Diagnostics Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Technology (CTC Detection and Enrichment Method, CTC Direct Detection Methods, and CTC Analysis), Application (Clinical/Liquid Biopsy and Research), and End User (Hospital and Clinics, Research and Academic Institutes, and Diagnostic Centers)
Historic Data: 2021-2024 | Base Year: 2025 | Forecast Period: 2026-2034- Report Date : Apr 2026
- Report Code : TIPRE00026639
- Category : Life Sciences
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
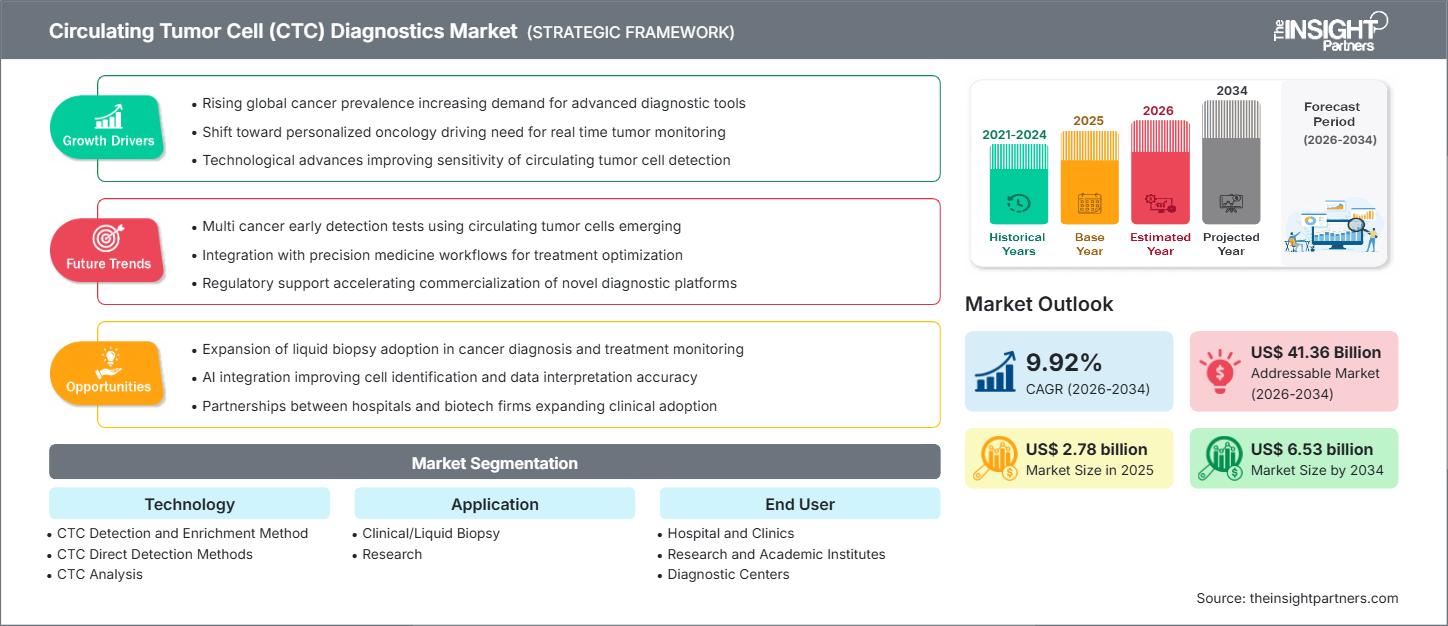
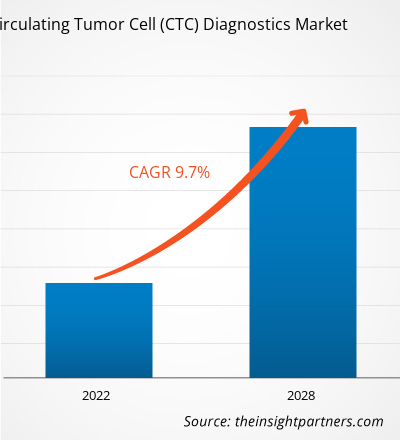
The circulating tumor cell (CTC) diagnostics market size is expected to reach US$ 6.53 billion by 2034 from US$ 2.78 billion in 2025. The market is anticipated to register a CAGR of 9.92% during 2026–2034.
Circulating Tumor Cell (CTC) Diagnostics Market Analysis
The Circulating Tumor Cell (CTC) Diagnostics market is expanding quickly, driven by the increasing global cancer incidence and the shift towards non-invasive cancer detection and monitoring. CTCs, shed from primary or metastatic tumors into the bloodstream, are a key component of liquid biopsy, offering valuable, real-time insights into tumor biology, heterogeneity, and metastatic potential without the need for traditional, invasive tissue biopsies. Technological advancements, particularly in microfluidics, immunomagnetic separation, and molecular analysis, have significantly improved the sensitivity and specificity of CTC isolation and characterization.
Circulating Tumor Cell (CTC) Diagnostics Market Overview
The implementation of Circulating Tumor Cell (CTC) diagnostics is revolutionizing cancer management. CTC analysis provides a non-invasive method for longitudinal monitoring of a patient's cancer status, enabling clinicians to track disease progression, detect minimal residual disease (MRD), and make timely, informed decisions about therapy adjustment. These techniques utilize various platforms, including microfluidic systems, immunocapture devices, and sophisticated analysis instruments. The data gathered from CTCs, such as molecular profiling, gene expression, and genetic mutations, helps to tailor treatment strategies and develop companion diagnostics, fulfilling the promise of precision oncology. The utility of CTCs extends across the patient journey, from early cancer screening and prognostic assessment to monitoring for recurrence and resistance mechanisms.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONCirculating Tumor Cell (CTC) Diagnostics Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Circulating Tumor Cell (CTC) Diagnostics Market Drivers and Opportunities
Market Drivers:
- Growing Global Cancer Prevalence: The increasing worldwide incidence of cancer, particularly among aging populations, fuels the demand for early, accurate, and repeatable diagnostic methods to improve survival rates.
- Shift Towards Non-Invasive Diagnostics (Liquid Biopsy): CTC diagnostics offer a minimally invasive alternative to traditional tissue biopsies, which are often painful and limited by tumor heterogeneity. The ease of collection allows for frequent monitoring of disease status.
- Advancements in Isolation and Detection Technologies: Ongoing innovations in microfluidics, label-free separation techniques, and high-sensitivity detection methods (e.g., molecular, imaging-based) are making CTC analysis more accurate, efficient, and clinically viable.
- Rising Demand for Personalized Medicine: CTC analysis provides dynamic molecular and genetic information about the tumor, which is crucial for identifying therapeutic targets, assessing drug resistance, and guiding personalized treatment selection.
Market Opportunities:
- Integration of AI and Machine Learning in Analysis: Utilizing Artificial Intelligence (AI) and Machine Learning (ML) can significantly improve the speed and accuracy of CTC identification, characterization, and molecular data interpretation, particularly for rare cells.
- Expansion of Clinical Utility for Early Screening and MRD: Generating robust clinical evidence through large-scale trials will enable the use of CTCs in early cancer screening for high-risk populations and for tracking Minimal Residual Disease (MRD), opening up vast new application segments.
- Development of Point-of-Care (POC) CTC Testing: Miniaturized, easy-to-use, and cost-effective POC CTC diagnostic devices would significantly expand access in clinical settings, particularly in emerging economies and community hospitals.
- Commercialization of CTC-Based Companion Diagnostics: Partnership between diagnostic companies and pharmaceutical firms to develop CTC-based tests that guide the use of specific targeted therapies (companion diagnostics) will secure regulatory approval and reimbursement.
Circulating Tumor Cell (CTC) Diagnostics Market Report Segmentation Analysis
The Circulating Tumor Cell (CTC) Diagnostics market share is analyzed across various segments to provide a clearer understanding of its structure, growth potential, and emerging trends. Below is a standard segmentation approach used in most industry reports:
By Technology:
- CTC Detection and Enrichment Methods
- CTC Direct Detection Methods
- CTC Analysis
By Application:
- Clinical/Liquid Biopsy
- Research
By End User:
- Hospitals and Clinics
- Research and Academic Institutes
- Diagnostic Centers
By Geography:
- North America
- Europe
- Asia-Pacific
- South & Central America
- Middle East & Africa
Circulating Tumor Cell (CTC) Diagnostics Market Regional Insights
The regional trends and factors influencing the Circulating Tumor Cell (CTC) Diagnostics Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Circulating Tumor Cell (CTC) Diagnostics Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Circulating Tumor Cell (CTC) Diagnostics Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2025 | US$ 2.78 billion |
| Market Size by 2034 | US$ 6.53 billion |
| Global CAGR (2026 - 2034) | 9.92% |
| Historical Data | 2021-2024 |
| Forecast period | 2026-2034 |
| Segments Covered |
By Technology
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Circulating Tumor Cell (CTC) Diagnostics Market Players Density: Understanding Its Impact on Business Dynamics
The Circulating Tumor Cell (CTC) Diagnostics Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Circulating Tumor Cell (CTC) Diagnostics Market top key players overview
Circulating Tumor Cell (CTC) Diagnostics Market Share Analysis by Geography
North America dominated the Circulating Tumor Cell (CTC) Diagnostics market. The dominance of this region is gaining traction due to its advanced healthcare infrastructure, high prevalence of cancer, significant government and private investment in precision oncology research, and the presence of leading technology developers and key opinion leaders.
The CTC Diagnostics market shows a different growth trajectory in each region due to factors such as cancer incidence rates, regulatory framework, and R&D spending. Below is a summary of market share and trends by region:
1. North America
- Market Share: Holds the highest market share (approx. 40-45%), driven by substantial research funding and quick adoption of advanced medical technologies.
- Key Drivers:
- High cancer incidence and prevalence.
- Strong government and private funding for cancer research and liquid biopsy.
- Presence of leading diagnostic and biopharmaceutical companies.
- Favorable reimbursement scenario for complex cancer diagnostics.
- Trends: Increasing integration of AI in CTC analysis, shift towards multiplexed assays, and a strong focus on clinical validation for therapy selection and monitoring.
2. Europe
- Market Share: Holds a significant market share, supported by a mature healthcare system and collaborative research efforts.
- Key Drivers:
- Increased awareness and clinical adoption of liquid biopsy.
- Public-private partnerships for oncology research.
- Government-led initiatives for cancer screening and early detection.
- Trends: Growing adoption of standardized CTC platforms, rising number of clinical trials incorporating CTC analysis, and expanding applications in personalized treatment stratification.
3. Asia Pacific
- Market Share: The fastest-growing regional market is anticipated, driven by a massive cancer patient pool and improving healthcare access.
- Key Drivers:
- Rapid modernization of healthcare infrastructure and increasing healthcare expenditure.
- Large, underserved cancer patient population, particularly in China and India.
- Supportive government policies promoting domestic biomedical research and technology adoption.
- Rising medical tourism for advanced cancer treatments.
- Trends: Focus on affordable, localized diagnostic solutions; significant R&D in microfluidics and molecular techniques; increasing collaborations between regional research institutes and global technology vendors.
4. South and Central America
- Market Share: Emerging region with moderate growth potential and rising adoption of advanced diagnostics.
- Key Drivers:
- Increasing cancer burden and improving awareness.
- Gradual improvement in digital and clinical infrastructure.
- Expansion of affordable diagnostic services by global and regional players.
- Trends: Initial adoption focused on prognostic assessment; increasing investment in establishing centralized molecular testing laboratories.
5. Middle East and Africa
- Market Share: Emerging market with strong growth potential, primarily led by countries with high healthcare spending.
- Key Drivers:
- Major national digital and healthcare transformation strategies (e.g., UAE, Saudi Arabia).
- Increasing investment in oncology centers of excellence.
- High prevalence of certain cancer types.
- Trends: Outsourcing of complex diagnostics to global labs; growing adoption of automated systems for large-scale screening and monitoring.
Circulating Tumor Cell (CTC) Diagnostics Market Players Density: Understanding Its Impact on Business Dynamics
The Circulating Tumor Cell (CTC) Diagnostics market is witnessing intensified competition due to the presence of both large, diversified life science corporations and specialized, innovative biotech startups. Companies are actively innovating to strengthen their market position and meet the growing demand for highly sensitive and specific liquid biopsy platforms across various clinical and research applications.
The competitive landscape is driving vendors to differentiate through:
- Developing next-generation platforms that offer higher purity, yield, and viability of isolated CTCs, such as advanced microfluidic and label-free enrichment systems.
- Enhancing the capability of CTC analysis systems to perform concurrent single-cell genomic, transcriptomic, and proteomic analysis to provide comprehensive biological insight.
- Investing heavily in large-scale clinical trials and obtaining regulatory approvals (e.g., FDA, CE-IVD) to standardize assays and integrate CTC testing into routine clinical guidelines.
Opportunities and Strategic Moves
- Large players are acquiring or partnering with specialized CTC technology startups to integrate proprietary isolation and detection methods, gaining a competitive edge and expanding their product portfolios.
- Collaborating with pharmaceutical companies to embed CTC monitoring into clinical trials for novel cancer drugs, positioning the CTC assay as a potential companion diagnostic.
- Targeting new application areas such as minimal residual disease (MRD) detection and treatment resistance monitoring, which offer high-growth potential beyond initial diagnostic use.
Major Companies Operating in the Circulating Tumor Cell (CTC) Diagnostics Market Are:
- Thermo Fisher Scientific Inc.
- STEMCELL Technologies Inc.
- QIAGEN
- Precision Medicine Group, LLC.
- Advanced Cell Diagnostics, Inc. (BIO-TECHNE CORPORATION)
- Epic Sciences
- ScreenCell
- Ikonisys, Inc.
- IVDiagnostics
Disclaimer: The companies listed above are not ranked in any particular order.
Circulating Tumor Cell (CTC) Diagnostics Market News and Recent Developments
- For instance, on June 02, 2025, QIAGEN announced the expansion of its oncology diagnostics portfolio with two strategic partnerships to advance the use of minimal residual disease (MRD) testing in clinical trials to support pharma co-development projects for companion diagnostics. The new collaborations with Tracer Biotechnologies and Foresight Diagnostics expand QIAGEN’s reach in MRD testing and cover solid tumors and hematological cancers.
Circulating Tumor Cell (CTC) Diagnostics Market Report Coverage and Deliverables
The "Circulating Tumor Cell (CTC) Diagnostics Market Size and Forecast (2021–2034)" report provides a detailed analysis of the market covering below areas:
- Circulating Tumor Cell (CTC) Diagnostics Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Circulating Tumor Cell (CTC) Diagnostics Market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Circulating Tumor Cell (CTC) Diagnostics Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments in the Circulating Tumor Cell (CTC) Diagnostics Market. Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For