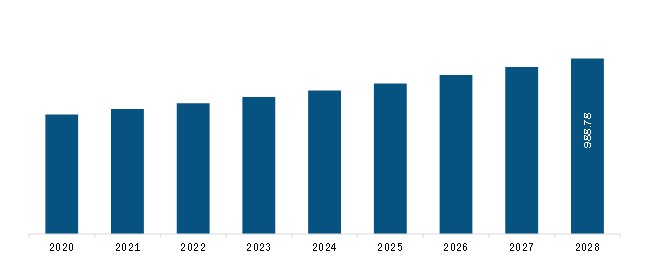
The Europe cerebrospinal fluid management market is expected to reach US$ 988.78 million by 2028 from US$ 694.04 million in 2021; it is estimated to grow at a CAGR of 5.2% from 2021 to 2028.
The growth of the market is attributed to rising technological developments for CSF shunts and growing developments for CSF management products. However, the risk associated with CSF shunts hinders the market growth.
The never-ending technological developments are the unstoppable trend for the growth of CSF management market. The risk of diagnosing and treatment failure, limited control options, and complications have offered various opportunities for the product developers. Rising advancements in technology have introduced the concept of “smart shunt” that could offer hi-tech control, diagnosis, and communication-based shunts or implants. It is anticipated that designed smart shunts can control CSF drainage based on feedback from one or more measured conditions. Additionally, researchers expect that smart shunts have the potential to offer sophisticated control over mechanical shunts—data with respect to shunt malfunction. It is further expected to reduce the risk of shunt obstructions and eventually reduce the risk of failures.
Late-stage development companies operating in the market are demanding experimental results to seek approvals and commercialize the smart shunts. The primary focus is on shunts designed to assess ICP noninvasively in the physician’s office through an implanted pressure sensor and noninvasive assessment of CSF flow in the clinical settings. These on-demand sensing systems will be a significantly advanced supplement to existing mechanical valves.
The growing demand for better treatments has resulted in various product developments, resulting in the Europe cerebrospinal fluid management market growth. For instance, in July 2018, FDA granted breakthrough device designation to F. Hoffmann-La Roche Ltd for its Elecsys ß-Amyloid (1-42) CSF and Elecsys Phospho-Tau (181P) CSF. The products are used as in-vitro diagnostic immunoassays to measure ß-Amyloid (1-42) and Phospho-Tau concentrations in CSF among adults with cognitive impairment. They are evaluated for Alzheimer’s disease (AD) and other causes of dementia.
The European economy is severely affected due to the exponential growth of COVID-19 cases in the region. Spain, Italy, Germany, France, and the UK are among the most-affected European countries. The COVID-19 emergency has undoubtedly aided in encouraging the general population to try out and expand their usage of digital services, particularly to avoid visiting a health center in person. However, there are still a number of constraints that hinder these services from becoming widely adopted.
Inconsistent and interrupted supply chain activities and unavailability of human resources negatively impacted the cerebrospinal fluid management market growth. The sudden onset of pandemic led to shutdown of neurological wards and clinics across numerous countries. At the same time, hospitals ran out of resources owing to a huge number of COVID-19 patients. Consequently, according to one study, many diagnostic and treatment procedures had been cancelled or postponed around the world.
Europe Cerebrospinal Fluid Management Market Revenue and Forecast to 2028 (US$ Mn)
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
EUROPE CEREBROSPINAL FLUID MANAGEMENT MARKET SEGMENTATION
By Product
- CSF shunts
- CSF drainage systems
By End User
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Neurological Centers
By Country
- U.K.
- Germany
- France
- Italy
- Spain
- Rest of Europe
Company Profiles
- Medtronic
- Integra Life Sciences Corporation
- B. Braun Melsungen AG
- DePuy Synthes
- Sophysa
- Dispomedica GmbH
- Möller Medical GmbH
- Natus Medical Incorporated
- Spiegelberg GmbH & Co. KG
Europe Cerebrospinal Fluid Management Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 694.04 Million |
| Market Size by 2028 | US$ 988.78 Million |
| Global CAGR (2021 - 2028) | 5.2% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | Europe
|
| Market leaders and key company profiles |
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends
Yes! We provide a free sample of the report, which includes Report Scope (Table of Contents), report structure, and selected insights to help you assess the value of the full report. Please click on the "Download Sample" button or contact us to receive your copy.
Absolutely - analyst assistance is part of the package. You can connect with our analyst post-purchase to clarify report insights, methodology or discuss how the findings apply to your business needs.
Once your order is successfully placed, you will receive a confirmation email along with your invoice.
• For published reports: You'll receive access to the report within 4-6 working hours via a secured email sent to your email.
• For upcoming reports: Your order will be recorded as a pre-booking. Our team will share the estimated release date and keep you informed of any updates. As soon as the report is published, it will be delivered to your registered email.
We offer customization options to align the report with your specific objectives. Whether you need deeper insights into a particular region, industry segment, competitor analysis, or data cut, our research team can tailor the report accordingly. Please share your requirements with us, and we'll be happy to provide a customized proposal or scope.
The report is available in either PDF format or as an Excel dataset, depending on the license you choose.
The PDF version provides the full analysis and visuals in a ready-to-read format. The Excel dataset includes all underlying data tables for easy manipulation and further analysis.
Please review the license options at checkout or contact us to confirm which formats are included with your purchase.
Our payment process is fully secure and PCI-DSS compliant.
We use trusted and encrypted payment gateways to ensure that all transactions are protected with industry-standard SSL encryption. Your payment details are never stored on our servers and are handled securely by certified third-party processors.
You can make your purchase with confidence, knowing your personal and financial information is safe with us.
Yes, we do offer special pricing for bulk purchases.
If you're interested in purchasing multiple reports, we're happy to provide a customized bundle offer or volume-based discount tailored to your needs. Please contact our sales team with the list of reports you're considering, and we’ll share a personalized quote.
Yes, absolutely.
Our team is available to help you make an informed decision. Whether you have questions about the report’s scope, methodology, customization options, or which license suits you best, we're here to assist. Please reach out to us at sales@theinsightpartners.com, and one of our representatives will get in touch promptly.
Yes, a billing invoice will be automatically generated and sent to your registered email upon successful completion of your purchase.
If you need the invoice in a specific format or require additional details (such as company name, GST, or VAT information), feel free to contact us, and we’ll be happy to assist.
Yes, certainly.
If you encounter any difficulties accessing or receiving your report, our support team is ready to assist you. Simply reach out to us via email or live chat with your order information, and we'll ensure the issue is resolved quickly so you can access your report without interruption.















- Medtronic
- Integra Life Sciences Corporation
- B. Braun Melsungen AG
- DePuy Synthes
- Sophysa
- Dispomedica GmbH
- Möller Medical GmbH
- Natus Medical Incorporated
- Spiegelberg GmbH & Co. KG






 Get Free Sample For
Get Free Sample For