Fetal Heart Rate Monitoring Devices Market Key Players, Opportunities, and Growth by 2031
Fetal Heart Rate Monitoring Devices Market Size and Forecasts (2021 - 2031), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage:By Product Type (Internal Fetal Heart Rate Monitoring, External Fetal Heart Rate Monitoring) ; Device Type (Doppler Ultrasound Device, Electronic Fetal Monitoring Device) ; Portability (Portable, Non-Portable) ; Method (Invasive, Noninvasive) ; End-User (Hospitals, Clinics, Home) , and Geography (North America, Europe, Asia Pacific, and South and Central America)
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Mar 2026
- Report Code : TIPRE00008622
- Category : Life Sciences
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
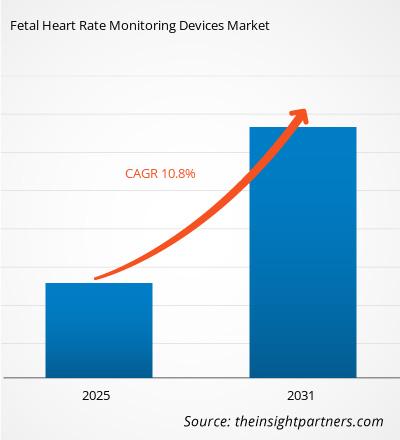
The Fetal Heart Rate Monitoring Devices Market is expected to register a CAGR of 10.8% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Product Type (Internal Fetal Heart Rate Monitoring, External Fetal Heart Rate Monitoring) . The report is segmented by Device Type (Doppler Ultrasound Device, Electronic Fetal Monitoring Device). The report is segmented by Portability (Portable, Non-Portable) . The report is segmented by Method (Invasive, Noninvasive). The report is segmented by End-User (Hospitals, Clinics, Home). The global analysis is further broken-down at regional level and major countries. The Report Offers the Value in USD for the above analysis and segments.
Purpose of the Report
The report Fetal Heart Rate Monitoring Devices Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Fetal Heart Rate Monitoring Devices Market Segmentation
Product Type
- Internal Fetal Heart Rate Monitoring
- External Fetal Heart Rate Monitoring
Device Type
- Doppler Ultrasound Device
- Electronic Fetal Monitoring Device
Portability
- Portable
- Non-Portable
Method
- Invasive
- Noninvasive
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONFetal Heart Rate Monitoring Devices Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Fetal Heart Rate Monitoring Devices Market Growth Drivers
- Technological Advancements: The constant new age of acute developments and innovations such as wireless monitoring and integration of smartphones for advanced data analytics has greatly driven the market for fetal heart rate monitoring devices. The new age monitors such as Doppler ultrasound and cardiotocography machines have replaced the traditional methods of monitoring and provided the healthcare providers with data that is accurate and in real time. Wearable fetal heart rate monitors along with other maternal health parameters, though, gain firm foothold since it's non-invasive and will be continuously monitored. These developments enhance the ability to provide high accuracy in assessments for a fetus but also improve the general experience of the user by both healthcare providers and expecting mothers.
- Increasing Awareness and Demand for Maternal Health: As public health campaigns and education continue to emphasize the need for maternal and fetal well-being, the visits for prenatal care have increased. Expectant mothers and healthcare providers have taken a keen interest in tracking the well-being of fetuses by checking fetal heart rates. The demand for fetal heart rate monitoring devices has been fueled by the sheer number of risky pregnancies over time. Health care providers are likely to invest in complex monitoring systems in order to ensure both mothers and their infants have better outcomes. Additionally, the shift towards preventive health care and proactive care has seen increased pregnancy monitoring, which advances the market even further.
- Rising Healthcare Expenditures: The growth of health expenditure is more evident in developing countries, which have increased their investment in maternal and infant health. Both the government and private operators are investing in the health infrastructure of their respective countries, thus easy access to sophisticated medical appliances, including fetal heart rate monitors. There is also an increased spending in the adoption of innovative healthcare technologies. With healthier expectancies for health care from the modern consumer, the demand for better facilities would lead to increased requirements for more reliable and effective fetal monitoring devices, hence a healthy market landscape.
Fetal Heart Rate Monitoring Devices Market Future Trends
- Shift Towards Non-Invasive Monitoring: Another observed trend is a shift to less invasive methods of fetal heart rate monitoring. Invasive techniques such as an internal fetal scalp electrode are painful to mothers and invasive. Doppler devices, wearable monitors, and others have found the grounds of adoption due to their popularity, comfort, and ease of use. Such a change is called for as the need to improve maternal experience becomes paramount as well as to reduce risks associated with invasive techniques. Manufacturers are focusing on designing non-invasive solutions with accurate real-time data as patient-centered care becomes more prevalent.
- Customization and Personalization of Devices: Customization is on a trend in medical devices as well; in the case of the fetal heart rate monitoring market, mothers-to-be have preferences and needs that are peculiar to them, and manufacturers have taken note of this by offering the ability to customize some features. These features would include comfort adjustability settings, choices to the aesthetic preference of designs, and bespoke reporting of fetal health data. This personalizes the experience toward the user's preference, enhancing further the user experience and bringing into play an even more emotional bonding between the expectant mothers and the monitoring devices which leads to higher acceptance and usage.
- Regulatory Focus on Safety and Efficacy: Assumed to be important by the regulatory authorities, the burgeoning market in fetal heart rate monitoring devices has required its players to walk the tightrope of tough regulation. Manufacturers have started to contend with strict regulatory requirements so that their products can meet high standards. The trend calls for increased spending on rigorous testing and clinical trials for validation of the devices by companies. Such emphasis on safety and efficacy thereby helps not only protect the patient but also give an upstanding value to manufacturers' reputations in the marketplace. Companies that get this right and emphasize regulatory compliance and the level of transparency stand a good chance of gaining a competitive edge.
Fetal Heart Rate Monitoring Devices Market Opportunities
- Integration of Artificial Intelligence (AI) and Big Data: With the integration of AI and big data analytics in the fetal monitoring device, it marks a very significant step towards innovation. AI algorithms can go through large datasets, help clinicians look for patterns, and predict some potential complications early in time. Predictive analytics could facilitate making right decisions with timely intervention by healthcare providers, thus ensuring better outcomes for both mothers and babies. For the investment that companies would make in developing AI: based solutions, they would be better positioned to attain a competitive advantage in the market as well as attract health facilities willing to resort to high- end prenatal care tools.
- Emerging Markets: Emerging markets will be huge growth drivers for the fetal heart rate monitoring devices market. Improving health care infrastructure and increased availability of prenatal care in regions like the Asia-Pacific, Latin America, and Middle East are raising demand for state: of: the: art medical devices. The opportunity that manufacturers can utilize here is that the products can be designed for these populations: affordability, portability, and ease of use. Collaboration with the local health provider and government efforts to promote maternal health may further open up markets in these regions.
- Telemedicine and Remote Monitoring: New avenues opened for fetal heart rate monitoring devices due to the growing need for telemedicine and remote monitoring solutions. Telemedicine got an instant boost by the pandemic caused by COVID 19 and various health care providers continue to extend service to expecting mothers through this medium, thus offering opportunities for the development of a home: based fetal monitoring system which continuously monitors and transfers data to the health care providers. These solutions may lead to higher participation by patients, fewer visits to hospitals, and reduced anxiety among expecting mothers. Companies that provide easy, reliable remote monitoring solutions can therefore have a considerable share of the market.
Fetal Heart Rate Monitoring Devices Market Regional Insights
The regional trends and factors influencing the Fetal Heart Rate Monitoring Devices Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Fetal Heart Rate Monitoring Devices Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Fetal Heart Rate Monitoring Devices Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 10.8% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Fetal Heart Rate Monitoring Devices Market Players Density: Understanding Its Impact on Business Dynamics
The Fetal Heart Rate Monitoring Devices Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Fetal Heart Rate Monitoring Devices Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Fetal Heart Rate Monitoring Devices Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Fetal Heart Rate Monitoring Devices Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
Frequently Asked Questions
1. Technological Advancements
2.Increasing Awareness and Demand for Maternal Health
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For