In Silico Trials Market Growth, Trends & Forecast 2034
In Silico Trials Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Organization Size (Small & Medium Organizations, and Large Organizations), Offerings (Products, Platforms, and Services), Application (Product Design & Discovery, Product Development, Pre-Clinical Targeting, Assessment of Drugs & Other Biomedical Products, and Others), Clinical Indication (Cardiovascular Diseases, Neurodegenerative Diseases, Oncology, Rare Diseases, Metabolic Diseases, Immune Based Diseases, Infectious Diseases, and Others), and End User (Pharmaceutical & Biopharmaceutical Companies, Medical Technology Companies, Contract Research Organizations, and Others)
Historic Data: 2021-2024 | Base Year: 2025 | Forecast Period: 2026-2034- Report Date : Apr 2026
- Report Code : TIPRE00027243
- Category : Technology, Media and Telecommunications
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
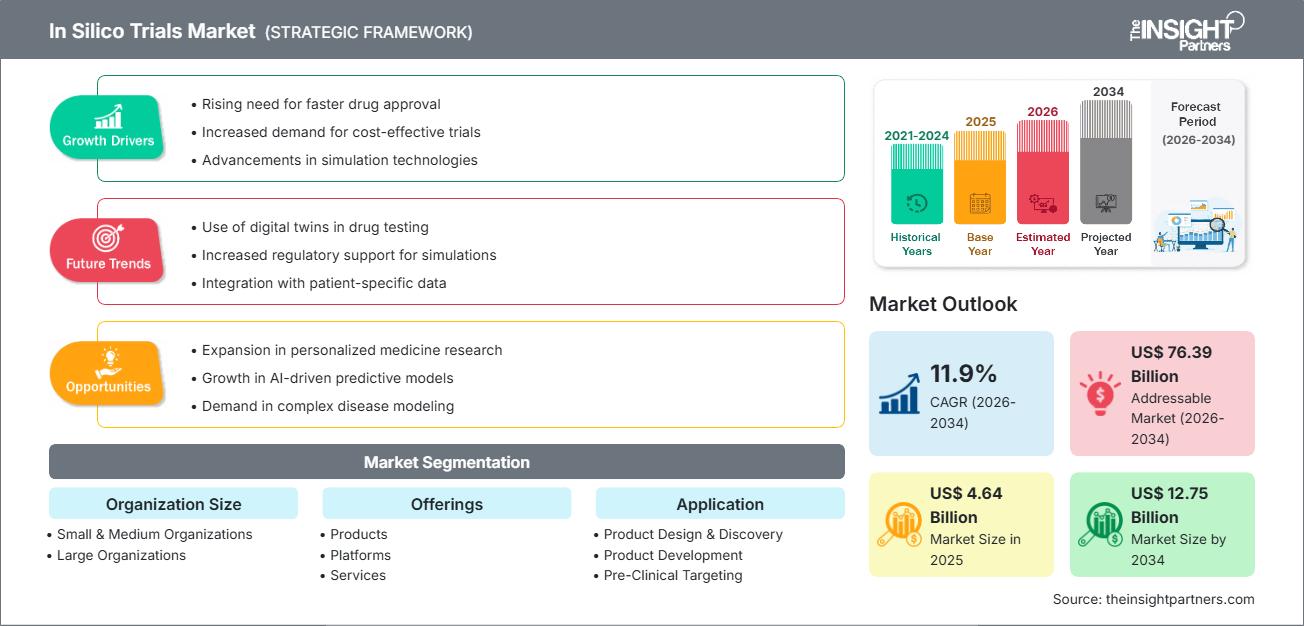
The in silico trials market size is expected to reach US$ 12.75 billion by 2034 from US$ 4.64 billion in 2025. The market is anticipated to register a CAGR of 11.9% during 2026–2034.
In Silico Trials Market Analysis
The in silico clinical trials market is expanding quickly, driven by the growing demand for faster drug and medical‑device development, the use of virtual patient populations, and the application of advanced simulation and modelling technologies. Companies are increasingly leveraging in silico methods to de-risk trials, optimise design, reduce cost and time, and improve regulatory acceptance of virtual evidence. The market is expected to grow significantly with increasing regulatory acceptance of virtual trials, the integration of real-world data into simulations, and the rise of personalised medicine and digital twin technologies.
In Silico Trials Market Overview
The implementation of in silico clinical trials helps companies increase efficiency and performance in drug and device development. In‑silico tools provide a deep understanding of biological systems, allow virtual patient modelling, simulate trial outcomes, de-risk design, and help regulatory submissions. For instance, simulations can identify failure modes, test extreme conditions, and optimise designs far more quickly and cost-effectively than purely physical trials. Currently, many pharmaceutical and MedTech companies and CROs are using in‑silico methods as part of their development workflow.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONIn Silico Trials Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
In Silico Trials Market Drivers and Opportunities
Market Drivers:
- Growing demand for faster drug/medical device development procedures and cost‑efficiencies.
- Rising adoption of virtual patient populations and digital twin technologies in clinical trials.
- Increasing regulatory acceptance of in silico evidence and simulation-based methods in trial and device approvals.
Market Opportunities:
- Expansion of simulation‑software and virtual‑cohort tools specifically tailored for pharmaceutical and medical‑device companies (cloud-based, scalable).
- Industry-specific in silico solutions for therapeutic areas such as oncology, neurology, and infectious diseases — enabling more precise trial simulations.
- Use of real-world data integration, AI/ML-powered simulations, and digital twin models to enhance trial design, reduce reliance on animal/physical models, and expedite regulatory submissions.
In Silico Trials Market Report Segmentation Analysis
The in silico trials market share is analyzed across various segments to provide a clearer understanding of its structure, growth potential, and emerging trends. Below is the standard segmentation approach used in most industry reports:
By Organization Size:
- Small & Medium Organizations: Adopt in-silico trials to reduce R&D costs, accelerate innovation, and compete efficiently with larger pharmaceutical and MedTech firms.
- Large Organizations: Utilize advanced simulation platforms for multi-phase virtual trials, regulatory submissions, and large-scale integration of AI-driven predictive modeling.
By Offerings:
- Products: Comprise software tools, digital twin models, and simulation kits that enable computational modeling of biological processes and virtual trials.
- Platforms: Cloud-based or on-premise ecosystems integrating AI, machine learning, and analytics for managing, simulating, and validating clinical trial data.
- Services: Include consulting, model validation, regulatory support, and outsourced simulation services for pharmaceutical and medical-device companies conducting in-silico trials.
By Application:
- Product Design & Discovery: Simulates early-stage molecular interactions to identify promising drug candidates or optimize device design before physical prototype development.
- Product Development: Applies computational modeling to refine dosage, efficacy, and safety parameters, accelerating regulatory approval and reducing time-to-market.
- Pre-Clinical Targeting: Uses virtual patient cohorts and AI models to test therapeutic hypotheses and predict outcomes prior to physical pre-clinical studies.
- Assessment of Drugs & Other Biomedical Products: Enables virtual testing of drug and device performance, safety, and interactions using digital twins and advanced simulation models.
By Clinical Indication
- Cardiovascular Diseases
- Neurodegenerative Diseases
- Oncology
- Rare Diseases
- Metabolic Diseases
- Immune-Based Diseases
- Infectious Diseases
By Region:
- North America
- Europe
- Asia‑Pacific
- Latin America (South & Central America)
- Middle East & Africa
In Silico Trials Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2025 | US$ 4.64 Billion |
| Market Size by 2034 | US$ 12.75 Billion |
| Global CAGR (2026 - 2034) | 11.9% |
| Historical Data | 2021-2024 |
| Forecast period | 2026-2034 |
| Segments Covered |
By Organization Size
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
In Silico Trials Market Players Density: Understanding Its Impact on Business Dynamics
The In Silico Trials Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
In Silico Trials Market Share Analysis by Geography
North America in‑silico clinical trials market led the global landscape, driven by rising demand for pharmaceutical drugs and medical devices, an increasing number of in‑silico trials, innovations in patient-centric solutions, the growth of remote or site-less clinical trials, and the emphasis on cost-efficient trial designs. Meanwhile, the Asia-Pacific market is projected to witness significant growth during the forecast period, fueled by evolving market dynamics, higher healthcare expenditures, and the growing adoption of in‑silico technologies, which offer immediate advantages such as streamlined workflows, improved data management, and enhanced decision-making capabilities.
The in silico trials market shows a different growth trajectory in each region due to factors such as efficient, cost-effective, and highly predictive tools for innovation and safety assessment. Below is a summary of market share and trends by region:
1. North America
- Market Share: Largest region in the global in silico clinical trials market, driven by strong R&D investment, regulatory frameworks, and presence of major players.
- Key Drivers: Adoption of advanced simulation technologies, presence of leading pharma/MedTech and CROs, and regulatory acceptance of in silico evidence.
- Trends: Increasing use of virtual patient cohorts, digital twin models, and cloud-based simulation platforms.
2. Europe
- Market Share: Significant growth, driven by regulatory support, increasing healthcare digitisation, and adoption of simulation-based trial methods.
- Key Drivers: Data‑privacy regulations, collaborations between public and private sectors in simulation research.
- Trends: Use of in silico modelling for cross-border clinical trials, multilingual virtual cohorts, and regulatory frameworks for modelling.
3. Asia‑Pacific
- Market Share: Fast-growing region due to increasing clinical trial activity, digital health adoption, and simulation technologies coming online.
- Key Drivers: Rising pharmaceutical/MedTech activity, governmental support for digital health and simulation research.
- Trends: Deployment of virtual patient models in India/China, regional hubs for simulation-based clinical trials.
4. Latin America (South & Central America)
- Market Share: Emerging region with growing interest in in silico trial methods among pharma/MedTech and CROs.
- Key Drivers: Increasing outsourcing of trial/simulation work, growth of regional CROs adopting simulation methods.
- Trends: Building local virtual‑cohort libraries, increased investment in simulation platforms.
5. Middle East & Africa
- Market Share: Emerging market with considerable potential, particularly in large-scale simulation projects and digital‑health transformation led by Gulf countries.
- Key Drivers: National strategies for digital health and simulation, demand for faster device/drug approval cycles.
- Trends: Use of in silico modelling for device approvals, local government-supported virtual‑trial initiatives.
In Silico Trials Market Players Density: Understanding Its Impact on Business Dynamics
The in silico trials market is witnessing intensified competition due to the presence of major global technology providers alongside emerging niche players and specialized startups. Companies are actively innovating to strengthen their market position and meet the growing demand for intelligent decision-making platforms across industries.
The competitive landscape is driving vendors to differentiate through:
- Development of platforms for virtual patient modelling, synthetic control arms, and digital twin technologies.
- Services that integrate real-world data, AI/ML analytics, and high-performance computing infrastructure for simulation accuracy.
- Adherence to regulatory standards and validation frameworks for in silico evidence to meet submissions to authorities like the U.S. Food and Drug Administration (FDA) and equivalent bodies globally.
Opportunities and Strategic Moves
- Pharma and MedTech companies are leveraging in silico trials to deliver more efficient, cost-effective, and patient-centric development, opening new value chains around simulation‑services and software.
- Through advanced simulation, virtual cohorts, digital twin models, and AI-powered prediction, in silico trials are enabling improved trial design, faster go/no‑go decisions, and reduced dependence on traditional physical trials.
- Major players, as well as startups, are collaborating or acquiring specialised simulation platforms to bring in capabilities such as virtual patient libraries, device simulation, and regulatory-ready modelling suites.
Major Companies Operating in the In Silico Trials Market Are:
- InSilico Trials Technologies
- Feops
- CADFEM Medical GmbH
- Dassault Systèmes
- Virtonomy GmbH
- Certara Inc.
- Computational Life
- NOVA
- TwInsight Medical
In Silico Trials Market News and Recent Developments
- For instance, on June 11, 2025, InSilicoTrials, a health tech company developing simulation-based solutions for drug development, announced its acceptance into the Microsoft for Startups Pegasus Program, an invitation-only initiative within Microsoft for Startups, designed to accelerate the growth of high-potential startups. This milestone marks a significant step in InSilicoTrials’ mission to modernize healthcare R&D through AI and in silico simulation technologies.
- In October 2024, Dassault Systèmes announced the availability of the world’s first guide for the medical device industry that outlines how to use virtual twins to accelerate clinical trials. This guide was published following the successful completion of a five-year collaboration with the U.S. Food and Drug Administration. The in silico clinical trial “ENRICHMENT Playbook” marks a significant advancement in the integration of virtual twins into the regulatory process in response to the need for improved patient safety, regulatory compliance, and pace of innovation.
In Silico Trials Market Report Coverage and Deliverables
The "In Silico Trials Market Size and Forecast (2021–2034)" report provides a detailed analysis of the market covering below areas:
- In Silico Trials Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- In Silico Trials Market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- In Silico Trials Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments in the In Silico Trials Market. Detailed company profiles
Frequently Asked Questions
Ankita is a dynamic market research and consulting professional with over 8 years of experience across the technology, media, ICT, and electronics & semiconductor sectors. She has successfully led and delivered 100+ consulting and research assignments for global clients such as Microsoft, Oracle, NEC Corporation, SAP, KPMG, and Expeditors International. Her core competencies include market assessment, data analysis, forecasting, strategy formulation, competitive intelligence, and report writing.
Ankita is adept at handling complete project cycles—from pre-sales proposal design and client discussions to post-sales delivery of actionable insights. She is skilled in managing cross-functional teams, structuring complex research modules, and aligning solutions with client-specific business goals. Her excellent communication, leadership, and presentation abilities have enabled her to consistently deliver value-driven outcomes in fast-paced and evolving market environments.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





 Get Free Sample For
Get Free Sample For