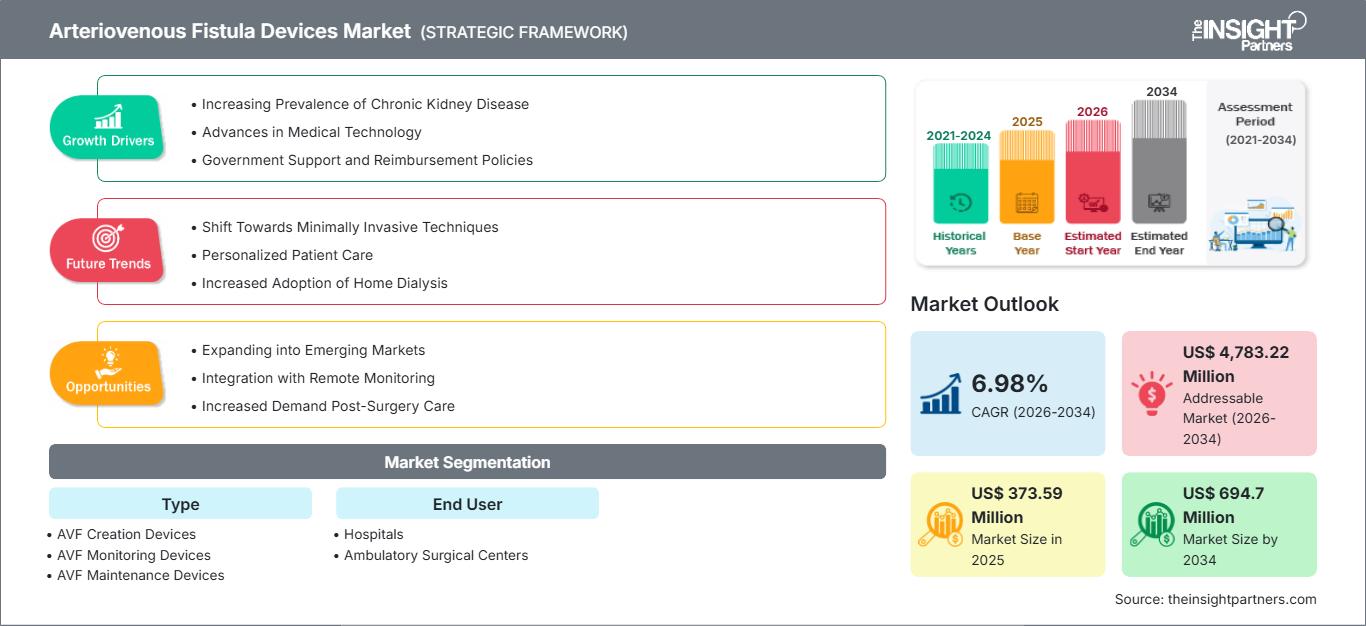
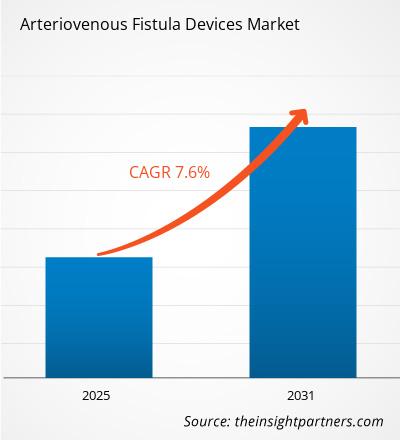
The global Arteriovenous Fistula Devices Market size is projected to reach US$ 694.7 Million by 2034 from US$ 373.59 Million in 2025. The market is anticipated to register a CAGR of 7.0% during the forecast period 2026–2034.
The report is segmented by Type (AVF Creation Devices, AVF Monitoring Devices, AVF Maintenance Devices).The report further presents analysis based on the End User (Hospitals, Ambulatory Surgical Centers, Others). The global analysis is further broken-down at regional level and major countries. The Report Offers the Value in USD for the above analysis and segments.
Purpose of the Report
The report Arteriovenous Fistula Devices Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Arteriovenous Fistula Devices Market Segmentation
Type
- AVF Creation Devices
- AVF Monitoring Devices
- AVF Maintenance Devices
End User
- Hospitals
- Ambulatory Surgical Centers
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONArteriovenous Fistula Devices Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Arteriovenous Fistula Devices Market Growth Drivers
- Increasing Prevalence of Chronic Kidney Disease: The increasing number of patients with chronic kidney disease (CKD) and the resultant need for dialysis has increased the demand for arteriovenous (AV) fistula devices. With AV fistulas being the preferred vascular access for dialysis, a growing number of patients who require dialysis is opening new avenues in the global market for AV fistula devices.
- Advances in Medical Technology: Innovation in vascular access devices, including improved materials, ease of use, and better patient outcomes, is contributing to market growth. These advancements help reduce complications associated with AV fistula procedures, enhancing patient satisfaction and leading to broader adoption among healthcare providers for dialysis access.
- Government Support and Reimbursement Policies: Many governments understand the significance of improved dialysis access and are providing financial incentives and better reimbursement policies to support the development and use of AV fistula devices. This policy ensures greater access to these lifesaving devices, thus catalyzing growth in these markets, especially in regions suffering from a high burden of kidney disease.
Arteriovenous Fistula Devices Market Future Trends
- Shift Towards Minimally Invasive Techniques: There is a drift toward minimally invasive techniques in making AV fistulas, that is, less invasive with quicker recovery time, fewer complications, and lower overall costs. When these procedures are more refined and less difficult to do, this becomes the preferred method of dialysis access and defines the development of new AV fistula devices.
- Personalized Patient Care: The dialysis care approach has been shifting toward individualized treatment plans, including customized AV fistula device choices for patients based on specific conditions such as vessel size, age, and comorbidities. This is fueling innovation in the design of AV fistula devices to better serve the diversity of patient needs and improve long-term outcomes.
- Increased Adoption of Home Dialysis: Increased uptake of home dialysis is promoting the use of more robust, convenient, and efficient AV fistula devices that will maintain the patient at home. Due to increased adoption in the home dialysis arena, this area creates an opportunity for developing ease-of-use, safe AV fistula devices for a highly autonomous patient, and an increased market reach and penetration.
Arteriovenous Fistula Devices Market Opportunities
- Expanding into Emerging Markets: Expanding into emerging markets is where potential exists because the incidences of chronic kidney diseases are growing, but few people there can access dialysis and AV fistula devices. Huge opportunities in this area exist for manufacturers and distributors to reach out for more people using these inexpensive devices.
- Integration with Remote Monitoring: Integration with remote monitoring technologies may offer an opportunity to track AV fistula device performance and patient health in real-time. Innovation of this type could be instrumental in the early detection of complications, improved management of dialysis patients, better outcomes for patients, and the opening of new avenues for the development of devices.
- Increased Demand Post-Surgery Care: There would be an increased demand in terms of post-surgery care and maintenance services for AV fistulas because patients require an increasingly significant portion of long-term management involving dialysis access. So creating that new revenue stream by having comprehensive service packages including devices monitoring, maintenance, or troubleshooting could create a positive environment for companies in the market to provide AV fistula device.
Arteriovenous Fistula Devices Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2025 | US$ 373.59 Million |
| Market Size by 2034 | US$ 694.7 Million |
| Global CAGR (2026 - 2034) | 6.98% |
| Historical Data | 2021-2024 |
| Forecast period | 2026-2034 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Arteriovenous Fistula Devices Market Players Density: Understanding Its Impact on Business Dynamics
The Arteriovenous Fistula Devices Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Arteriovenous Fistula Devices Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Arteriovenous Fistula Devices Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
Frequently Asked Questions
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends







 Get Free Sample For
Get Free Sample For