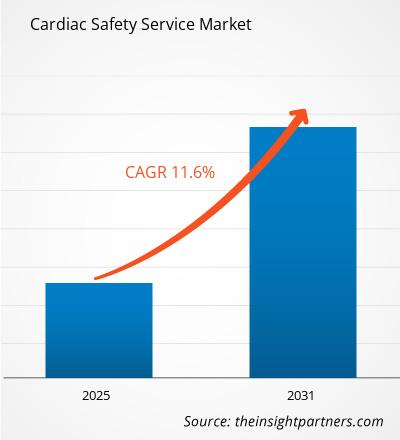
The Cardiac Safety Service Market is expected to register a CAGR of 11.6% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Type (Standalone, Integrated). The report further presents analysis based on the Services (ECG/Holter Measurement, Blood Pressure Measurement, Cardiac Imaging, Thorough QT Studies), End User (Pharmaceutical & Biopharma, CROs). The global analysis is further broken-down at regional level and major countries. The Report Offers the Value in USD for the above analysis and segments.
Purpose of the Report
The report Cardiac Safety Service Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Cardiac Safety Service Market Segmentation
Type
- Standalone
- Integrated
Services
- ECG/Holter Measurement
- Blood Pressure Measurement
- Cardiac Imaging
- Thorough QT Studies
End User
- Pharmaceutical & Biopharma
- CROs
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONCardiac Safety Service Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Cardiac Safety Service Market Growth Drivers
- Rising Prevalence of Cardiovascular Diseases: Cardiovascular diseases (CVDs) remain the leading cause of death globally, which directly impacts the demand for cardiac safety services. With an increasing number of patients undergoing clinical trials for new cardiovascular drugs and therapies, the need for rigorous cardiac safety assessments has become crucial. Regulatory agencies, such as the FDA and EMA, require extensive cardiac safety data before approving new medications, particularly those with potential cardiovascular side effects. As pharmaceutical companies focus on CVD treatments, the demand for specialized cardiac safety services continues to rise, ensuring drugs meet safety standards for heart health.
- Regulatory Stringency: Regulatory agencies around the world, particularly the FDA, have tightened their scrutiny of drugs that may have adverse effects on the cardiovascular system. This has led to an increased need for cardiac safety services during clinical trials. To ensure compliance with regulatory standards, drug developers rely on specialized testing and monitoring services to evaluate potential risks to the heart, such as arrhythmias or QT interval prolongation. This need for comprehensive cardiac safety assessments in the early stages of drug development is creating significant demand for specialized service providers in the field.
- Advancements in Cardiac Monitoring Technologies: Advances in cardiac monitoring technologies, including telemetry, ECG, and wearable devices, are driving the cardiac safety services market. These technologies provide real-time, continuous monitoring of heart function during clinical trials and patient treatment. They allow for more accurate and detailed assessments of cardiac risks, particularly for drugs that may cause heart arrhythmias or other cardiovascular issues. As these technologies continue to evolve, they enable service providers to offer more precise cardiac safety monitoring, improving drug safety profiles and regulatory compliance, thus fueling market growth.
Cardiac Safety Service Market Future Trends
- Personalized Cardiac Safety Assessments: With the increasing focus on personalized medicine, there is a growing trend toward individualized cardiac safety assessments in clinical trials. Instead of applying generic standards, clinical trials are moving toward more tailored approaches based on patients’ unique genetic profiles, comorbidities, and specific cardiac conditions. By integrating genetic and clinical data, service providers can offer more precise cardiac safety evaluations, ensuring better safety profiles for drugs. This trend is being driven by advancements in genomics and precision medicine, making cardiac safety services more sophisticated and individualized.
- Integration of AI and Big Data: The integration of artificial intelligence (AI) and big data analytics into cardiac safety services is transforming the market. AI algorithms are being used to analyze vast amounts of cardiovascular data from clinical trials, improving the speed and accuracy of identifying potential heart-related risks. By processing large datasets from ECGs, telemetry, and other monitoring tools, AI can detect patterns that might be overlooked by human analysts. This trend is leading to faster, more accurate cardiac safety assessments and has the potential to revolutionize how drugs are tested for heart-related risks.
- Telemedicine and Remote Cardiac Monitoring: The adoption of telemedicine and remote monitoring technologies is becoming a significant trend in the cardiac safety services market. With the increased use of wearable devices and mobile health apps, patients can now be monitored remotely during clinical trials or ongoing treatments. This approach not only offers convenience but also allows for continuous monitoring, ensuring that any cardiovascular events are detected in real-time. Remote cardiac monitoring is expected to expand further, driven by the growing demand for cost-effective, patient-centric care and regulatory pressure to ensure comprehensive safety during clinical trials.
Cardiac Safety Service Market Opportunities
- Growth of Clinical Trials in Emerging Markets: Emerging markets, particularly in Asia-Pacific and Latin America, are becoming key hubs for clinical trials due to their large patient populations and growing healthcare infrastructure. As pharmaceutical companies expand their clinical trial operations into these regions, the demand for cardiac safety services will increase. Service providers that specialize in cardiovascular safety assessments can capitalize on this growth by offering tailored services that comply with local regulatory requirements while maintaining global standards. These regions represent a significant opportunity for market expansion and service innovation.
- Expansion of Drug Development for Cardiovascular Diseases: As pharmaceutical companies increasingly focus on developing new treatments for cardiovascular diseases, the demand for specialized cardiac safety services will continue to grow. New cardiovascular drugs, biologics, and gene therapies require rigorous cardiac safety monitoring during clinical trials. Service providers that offer advanced monitoring solutions, such as continuous ECG, telemetry, and AI-based analysis, will be well-positioned to meet the evolving needs of the industry. This presents an opportunity for service providers to expand their offerings and partner with pharmaceutical companies developing next-generation cardiovascular treatments.
- Regulatory and Compliance Changes: As regulatory bodies continue to update and tighten cardiac safety guidelines, there will be greater demand for specialized services that help companies navigate these evolving requirements. Regulatory bodies like the FDA and EMA have been increasing their focus on the cardiovascular safety of new drugs. Companies that provide comprehensive, up-to-date expertise in compliance with these changing regulations can seize this opportunity to offer invaluable support to pharmaceutical companies. By ensuring that clinical trials meet the latest standards, these service providers can capture a larger share of the growing market.
Cardiac Safety Service Market Regional Insights
The regional trends and factors influencing the Cardiac Safety Service Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Cardiac Safety Service Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Cardiac Safety Service Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 11.6% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Cardiac Safety Service Market Players Density: Understanding Its Impact on Business Dynamics
The Cardiac Safety Service Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Cardiac Safety Service Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Cardiac Safety Service Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Cardiac Safety Service Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
Frequently Asked Questions
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For