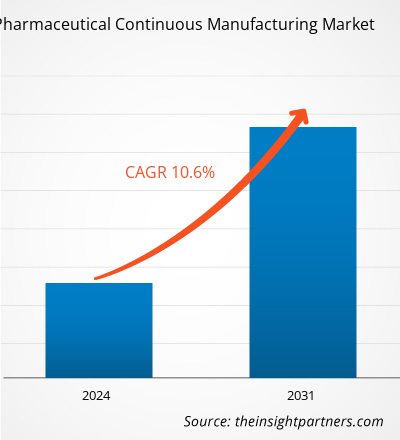
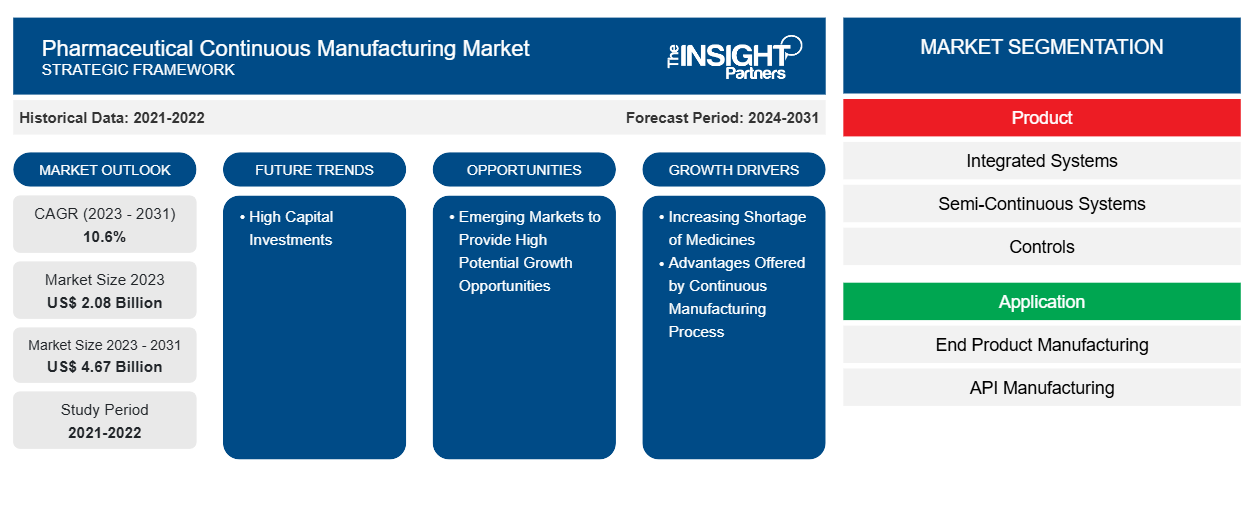
Se prevé que el tamaño del mercado de fabricación continua farmacéutica alcance los 4.670 millones de dólares en 2031, frente a los 2.080 millones de dólares en 2023. Se espera que el mercado registre una CAGR del 10,6 % durante el período 2023-2031. Es probable que el creciente apoyo de los organismos reguladores, la creciente adopción por parte de los fabricantes contratados y de la propia empresa de procedimientos de fabricación de medicamentos y las ventajas asociadas a la fabricación continua sigan siendo tendencias clave en el mercado.
Análisis del mercado de fabricación continua farmacéutica
Las empresas farmacéuticas invierten constantemente en tecnologías de fabricación innovadoras para obtener una ventaja competitiva y mantenerse a la vanguardia del mercado, impulsando así el mercado de la fabricación continua. La fabricación continua permite a las empresas farmacéuticas tener un mayor control sobre su proceso de producción, lo que ayuda a mejorar la calidad y la consistencia del producto final, reduciendo así las desviaciones, el desperdicio y ahorrando tiempo de retrabajo y costos de recuperación. La fabricación continua farmacéutica puede mejorar la calidad del producto final, mejorar el control del proceso y permitir pruebas de liberación en tiempo real. La Agencia Europea de Medicamentos (EMA) y la Administración de Alimentos y Medicamentos de los Estados Unidos (FDA) son los organismos reguladores que cada vez favorecen más el uso de la fabricación continua en la industria farmacéutica. Además, la fabricación continua se ha vuelto más atractiva y práctica en la industria farmacéutica debido a la mejora de la supervisión, el control y la optimización del proceso que se proporciona a través de los medios de automatización, control del sistema, análisis de datos y tecnología de análisis de procesos (PAT), que impulsa el mercado.
Descripción general del mercado de fabricación continua farmacéutica
Como solución viable para minimizar la presión y reducir el tiempo y los costos de desarrollo de medicamentos, manteniendo al mismo tiempo la calidad y el suministro del producto final en la industria farmacéutica, la fabricación continua se está utilizando ampliamente. Por lo tanto, debido a estos beneficios, el mercado de fabricación continua está experimentando un crecimiento saludable. Además, las crecientes iniciativas para promover el uso de sistemas de fabricación farmacéutica continua por parte de la Administración de Alimentos y Medicamentos (FDA) y las numerosas ventajas de los sistemas de fabricación continua sobre la fabricación por lotes son algunos de los principales factores que impulsan el crecimiento del mercado. Sin embargo, por otro lado, es probable que el alto costo de implementación de los sistemas de fabricación continua farmacéutica obstaculice el crecimiento del mercado.
Personalice este informe según sus necesidades
Obtendrá personalización en cualquier informe, sin cargo, incluidas partes de este informe o análisis a nivel de país, paquete de datos de Excel, así como también grandes ofertas y descuentos para empresas emergentes y universidades.
-
Obtenga las principales tendencias clave del mercado de este informe.Esta muestra GRATUITA incluirá análisis de datos, desde tendencias del mercado hasta estimaciones y pronósticos.
Factores impulsores y oportunidades del mercado de fabricación continua farmacéutica
Ventajas que ofrece el proceso de fabricación continua para favorecer el mercado
La fabricación continua farmacéutica está ganando cada vez más importancia debido a la creciente demanda de productos biológicos y alternativas de fabricación flexibles. Las empresas que están haciendo la transición a los procedimientos de fabricación continua están obteniendo rápidamente las aprobaciones de la FDA. El proceso de fabricación continua ofrece muchas ventajas, entre las que se incluyen la eficiencia en el tiempo, la reducción de las necesidades y el desperdicio de energía y el aumento de la productividad. Además, el proceso reduce el riesgo de error humano debido a la participación de pocas personas en el proceso de fabricación. Por lo tanto, debido a las ventajas mencionadas anteriormente que ofrece la fabricación continua, se espera que el mercado crezca en los próximos años.
Los mercados emergentes ofrecerán oportunidades de crecimiento con alto potencial
En regiones como Asia Pacífico y el Sur y Centro están surgiendo lugares atractivos para la subcontratación de las industrias biofarmacéuticas . China e India ofrecen bajos costos de fabricación y operación que son factores importantes para impulsar el crecimiento del mercado en la región de Asia Pacífico. Tanto China como India indican perspectivas positivas para el futuro del mercado debido al reciente crecimiento de la industria biofarmacéutica en ambos países. En enero de 2020, STA Pharmaceutical Co., Ltd., una subsidiaria de WuXi AppTec abrió una nueva planta de fabricación de ingredientes farmacéuticos activos (API) de oligonucleótidos a gran escala en China. Por lo tanto, es probable que los mercados emergentes adecuados para la industria biofarmacéutica actúen como generadores de ingresos y crecimiento de alto potencial en el mercado de fabricación continua farmacéutica.
Informe de mercado de fabricación continua farmacéutica Análisis de segmentación
Los segmentos clave que contribuyeron a la derivación del análisis del mercado de fabricación continua farmacéutica son el producto, la aplicación y el usuario final.
- Según el producto, el mercado de fabricación continua farmacéutica se segmenta en sistemas integrados, sistemas semicontinuos y controles. El segmento de sistemas integrados tuvo la mayor participación de mercado en 2023 y se espera que registre la CAGR más alta durante el período de pronóstico.
- Por aplicación, el mercado se divide en fabricación de productos finales y fabricación de API . El segmento de fabricación de productos finales se divide a su vez en fabricación de dosis sólidas y fabricación de dosis líquidas. El segmento de fabricación de productos finales tuvo la mayor participación del mercado en 2023. Sin embargo, se prevé que el segmento de fabricación de API registre la CAGR más alta durante 2021-2031.
- Según el usuario final, el mercado de fabricación continua farmacéutica se divide en empresas de fabricación a gran escala y departamentos de I+D. El segmento de fabricación a gran escala tuvo la mayor participación de mercado en 2023 y se espera que registre la CAGR más alta durante el período de pronóstico.
Análisis de la cuota de mercado de la fabricación continua farmacéutica por geografía
El alcance geográfico del informe de mercado de fabricación continua farmacéutica se divide principalmente en cinco regiones: América del Norte, Asia Pacífico, Europa, Medio Oriente y África, y América del Sur y Central.
Norteamérica ha dominado el mercado de fabricación continua farmacéutica. Los factores que han llevado al crecimiento de este mercado en Norteamérica se deben al creciente apoyo de la FDA a la promoción del uso de la fabricación continua en lugar de la fabricación por lotes, la creciente escasez de medicamentos en la región y la presencia de gigantes farmacéuticos que pueden permitirse las grandes inversiones iniciales necesarias para establecer procesos de fabricación continua. La mayoría de las industrias de los EE. UU. han adoptado procesos de fabricación continua desde hace décadas. Estados Unidos mantiene una posición dominante en Norteamérica debido a factores como el creciente número de empresas farmacéuticas que adoptan procedimientos de fabricación continua, los avances en el campo de las tecnologías de fabricación y la eficiencia que ofrecen estas configuraciones para aumentar los volúmenes de producción. Además, la FDA de los EE. UU. emprendió varias iniciativas para promover la fabricación continua en los EE. UU. dentro de las industrias farmacéuticas.
Perspectivas regionales del mercado de fabricación continua farmacéutica
Los analistas de Insight Partners explicaron en detalle las tendencias y los factores regionales que influyen en el mercado de fabricación continua farmacéutica durante el período de pronóstico. Esta sección también analiza los segmentos y la geografía del mercado de fabricación continua farmacéutica en América del Norte, Europa, Asia Pacífico, Oriente Medio y África, y América del Sur y Central.
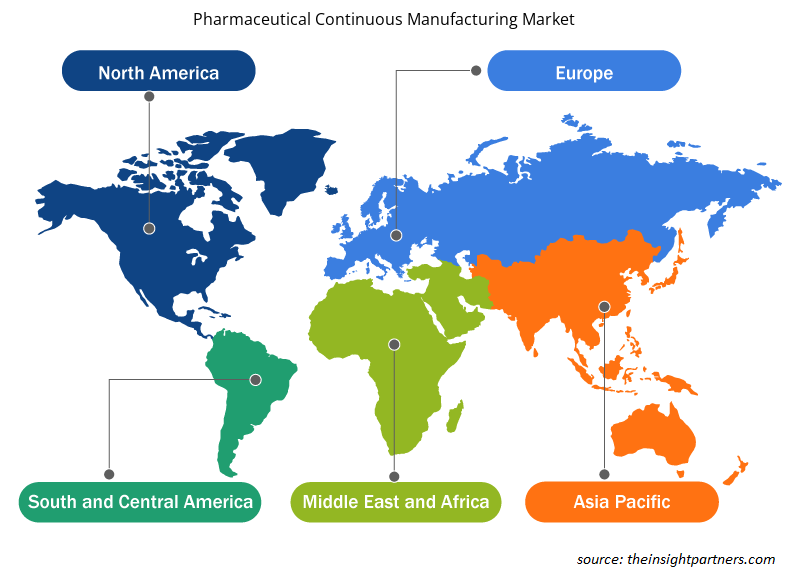
- Obtenga datos regionales específicos para el mercado de fabricación continua farmacéutica
Alcance del informe de mercado de fabricación continua farmacéutica
| Atributo del informe | Detalles |
|---|---|
| Tamaño del mercado en 2023 | US$ 2.08 mil millones |
| Tamaño del mercado en 2031 | US$ 4.67 mil millones |
| CAGR global (2023 - 2031) | 10,6% |
| Datos históricos | 2021-2022 |
| Período de pronóstico | 2024-2031 |
| Segmentos cubiertos |
Por producto
|
| Regiones y países cubiertos |
América del norte
|
| Líderes del mercado y perfiles de empresas clave |
|
Densidad de actores del mercado: comprensión de su impacto en la dinámica empresarial
El mercado de fabricación continua farmacéutica está creciendo rápidamente, impulsado por la creciente demanda de los usuarios finales debido a factores como la evolución de las preferencias de los consumidores, los avances tecnológicos y una mayor conciencia de los beneficios del producto. A medida que aumenta la demanda, las empresas amplían sus ofertas, innovan para satisfacer las necesidades de los consumidores y aprovechan las tendencias emergentes, lo que impulsa aún más el crecimiento del mercado.
La densidad de actores del mercado se refiere a la distribución de las empresas o firmas que operan dentro de un mercado o industria en particular. Indica cuántos competidores (actores del mercado) están presentes en un espacio de mercado determinado en relación con su tamaño o valor total de mercado.
Las principales empresas que operan en el mercado de fabricación continua farmacéutica son:
- Grupo GEA
- Coperion GmbH
- Gericke AG
- Glatt GmbH
- Grupo Hosokawa Micron
- Compañía de maquinaria Munson Inc.
Descargo de responsabilidad : Las empresas enumeradas anteriormente no están clasificadas en ningún orden particular.
- Obtenga una descripción general de los principales actores clave del mercado de fabricación continua farmacéutica
Noticias y desarrollos recientes del mercado de fabricación continua farmacéutica
El mercado de fabricación continua farmacéutica se evalúa mediante la recopilación de datos cualitativos y cuantitativos posteriores a la investigación primaria y secundaria, que incluye publicaciones corporativas importantes, datos de asociaciones y bases de datos. A continuación, se enumeran algunos de los desarrollos en el mercado de fabricación continua farmacéutica:
- SK Bioscience, una organización de desarrollo y fabricación por contrato con sede en Corea del Sur (CDMO), ha iniciado la construcción de una ampliación de aproximadamente 4200 m2 de su planta de fabricación de vacunas L House en Andong, Gyeongsangbuk-do, Corea del Sur, que servirá como base de producción para la vacuna conjugada antineumocócica candidata GBP410, desarrollada conjuntamente por SK Bioscience y Sanofi, que están coinvirtiendo en la ampliación. (SK Bioscience, comunicado de prensa, marzo de 2024)
- Phlow Corp., una empresa de beneficio público de medicamentos esenciales con sede en EE. UU., anunció alianzas estratégicas con el Instituto de Medicamentos para Todos de la Universidad Commonwealth de Virginia (VCU) y con AMPAC Fine Chemicals para brindar servicios de investigación y desarrollo (I+D) de fabricación continua por contrato para productos farmacéuticos de moléculas pequeñas. Además de sus sólidas y crecientes capacidades internas, la red de socios estratégicos establecidos, innovadores y experimentados de Phlow brindará soluciones de fabricación avanzada de alta calidad con sede en EE. UU. para ingredientes farmacéuticos activos (API) de moléculas pequeñas y materiales de partida registrados (RSM) en todas las etapas de desarrollo utilizando tecnologías de vanguardia y conocimientos únicos de la industria. (Phlow Corp., Noticias, marzo de 2022)
Cobertura y resultados del informe sobre el mercado de fabricación continua farmacéutica
El informe “Tamaño y pronóstico del mercado de fabricación continua farmacéutica (2021-2031)” proporciona un análisis detallado del mercado que cubre las siguientes áreas:
- Tamaño y pronóstico del mercado de fabricación continua farmacéutica a nivel global, regional y nacional para todos los segmentos clave del mercado cubiertos bajo el alcance
- Tendencias del mercado de fabricación continua farmacéutica, así como dinámica del mercado, como impulsores, restricciones y oportunidades clave
- Análisis PEST y FODA detallados
- Análisis del mercado de fabricación continua farmacéutica que abarca las tendencias clave del mercado, el marco global y regional, los principales actores, las regulaciones y los desarrollos recientes del mercado.
- Análisis del panorama de la industria y de la competencia que abarca la concentración del mercado, el análisis de mapas de calor, los actores destacados y los desarrollos recientes para el mercado de fabricación continua farmacéutica
- Perfiles detallados de empresas
- Análisis histórico (2 años), año base, pronóstico (7 años) con CAGR
- Análisis PEST y FODA
- Tamaño del mercado, valor/volumen: global, regional y nacional
- Industria y panorama competitivo
- Conjunto de datos de Excel
Informes recientes
Testimonios
Razón para comprar
- Toma de decisiones informada
- Comprensión de la dinámica del mercado
- Análisis competitivo
- Información sobre clientes
- Pronósticos del mercado
- Mitigación de riesgos
- Planificación estratégica
- Justificación de la inversión
- Identificación de mercados emergentes
- Mejora de las estrategias de marketing
- Impulso de la eficiencia operativa
- Alineación con las tendencias regulatorias






















 Obtenga una muestra gratuita para - Mercado de fabricación continua farmacéutica
Obtenga una muestra gratuita para - Mercado de fabricación continua farmacéutica