Finished Dosage Forms Segment Drives Urokinase API and Finished Dosage Forms Market Growth
According to our new research study named "Urokinase API and Finished Dosage Forms Market Forecast to 2031 – Global Analysis – by Product Type, Manufacturing Process, Indication, Distribution Channel, and Geography," the market was valued at US$ 2.37 billion in 2024 and is projected to reach US$ 3.60 billion by 2031; it is expected to register a CAGR of 6.3% during 2025–2031. The rising occurrence of thromboembolic disorders, surging geriatric population, and advancements in manufacturing techniques to improve yield drive the adoption of urokinase. Advancements in biosimilars and recombinant API are projected to bring new urokinase API and finished dosage forms market trends in the coming years.
Urokinase is an enzyme utilized as a thrombolytic agent to dissolve blood clots. It is biologically sourced from human kidney cells or through recombinant technology. It is a protein-based drug, thus its production and storage need special measures.
Urokinase API and Finished Dosage Forms Market, by Region, 2024 (%)
Urokinase API and Finished Dosage Forms Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product Type (Active Pharmaceutical Ingredient and Finished Dosage Forms), Manufacturing Process (Urine-derived, Cell Culture-based, and Recombinant Technology), Indication (Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), Catheter Occlusion, Myocardial Infarction (Heart Attack), Peripheral Arterial Occlusive Diseases, and Others), Distribution Channel (Hospital Pharmacies, Retail and Drug Stores, Direct Sales (API and FDF), and Online Pharmacies), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)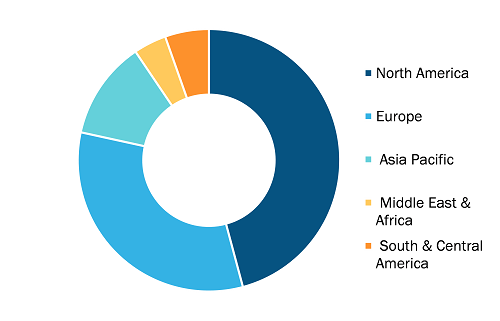
Urokinase API and Finished Dosage Forms Market Forecast 2031
Download Free Sample
Source: The Insight Partners Analysis
The rising number of cases of heart diseases, strokes, and pulmonary embolism, mainly among the elderly population, is driving global urokinase API and finished dosage forms market growth. The rise in surgeries requiring the eliminating of the risk of thrombosis post-operation contributes to the demand. The authorization by regulators and the increased use of biosimilars facilitate access, contributing to the rising urokinase API and finished dosage forms market size.
Urokinase API and Finished Dosage Forms Market Analysis Based on Segmental Evaluation:
Based on product type, the urokinase API and finished dosage forms market is bifurcated into API and finished dosage forms. The finished dosage forms segment held a significant urokinase API and finished dosage forms market share in 2024. Finished Dosage Forms (FDF) are the same as the pharmaceutical industry's end products that the patients can use right away. Consequently, the drug urokinase is fully finished in formulation, packaging, and labeling for clinical use. It is packaged in vials containing dosages ranging from 10,000 to 500,000 IU or more, requiring reconstitution with sterile saline or water before intravenous injection or infusion. The technology in aseptic manufacturing, packaging, and the quality of the vial extend the shelf life and reliability of solution forms, which have become more favored in high-stress, acute care environments. Syner-Kinase by Syner-Med Ltd. obtained the UK and the EMA approvals in 2019. It is available as a pre-dissolved solution for infusion, making it possible for continuous intravenous delivery over hours so that fibrinolytic effects are optimal and the risk of bleeding is lowered. The biotech companies producing recombinant stable quick-administration and indication-broadened FDFs are better positioned to capture a large market share by catering to various demands.
The geographical scope of the urokinase API and finished dosage forms market report includes the assessment of the market performance in North America, Europe, Asia Pacific, South and Central America, and the Middle East and Africa. North America dominated the urokinase API and finished dosage forms market share in 2024. The rising prevalence of cardiovascular diseases, including the cases of deep vein thrombosis and pulmonary embolism, is driving in the urokinase API and finished dosage forms market growth in North America. This region is among the most benefited by pharmaceutical research and development, in which companies with large capital are introducing next-generation products based on urokinase. The administration's support and the rapid approval by organizations such as the US FDA are propelling the market entry of the new formulations of urokinase, contributing to the urokinase API and finished dosage forms market growth. The pharmaceutical industry's strategy of collaborating with biosciences for technology implantation and the use of biological manufacturing in producing pharma products are two of the most outstanding factors behind their expansion into the market segment. The US population is aging very quickly, leading to an increase in the incidence of cardiovascular diseases (CVDs) and thrombotic disorders. This demographic trend encourages corporations to invest in synthetic urokinase API by developing recombinant methods that ensure a stable and reliable supply chain. Therefore, the above-mentioned factors support the growth of the global urokinase API and finished dosage forms market.
Taj Pharmaceuticals Ltd, Aetos Pharma Private Limited, Ilex Life Sciences, Jiangxi Haoran Bio-Pharma Co., Ltd., Microbix Biosystems, Syner-Med (Pharmaceutical Products) Ltd., Midas Pharma GmbH, Cerbios-Pharma SA, Fresenius Kabi AG, and Cadila Pharmaceuticals Ltd. are among the leading companies profiled in the urokinase API and finished dosage forms market report.
Based on product type, the urokinase API and finished dosage forms market is segmented into active pharmaceutical ingredients and finished dosage forms. Based on the manufacturing process, the market is classified into urine-derived, cell culture-based, and recombinant technology. Based on indication, the urokinase API and finished dosage forms market is segmented into deep vein thrombosis and pulmonary embolism, catheter occlusion, myocardial infarction (heart attack), peripheral arterial occlusive diseases, and others. By distribution channel, the market is categorized into hospital pharmacies, retail and drug stores, direct sales (API and FDF), and online pharmacies. Geographically, the urokinase API and finished dosage forms market is categorized into North America (US, Canada, and Mexico), Europe (France, Germany, UK, Spain, Italy, and the Rest of Europe), Asia Pacific (China, Japan, India, South Korea, Australia, and the Rest of Asia Pacific), the Middle East and Africa (Saudi Arabia, South Africa, the UAE, and the Rest of the Middle East and Africa), and South and Central America (Brazil, Argentina, and the Rest of South and Central America).
Contact Us
Phone: +1-646-491-9876
Email Id: sales@theinsightpartners.com