Urokinase API and Finished Dosage Forms Market Trends, and Opportunities by 2031
Urokinase API and Finished Dosage Forms Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product Type (Active Pharmaceutical Ingredient and Finished Dosage Forms), Manufacturing Process (Urine-derived, Cell Culture-based, and Recombinant Technology), Indication (Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), Catheter Occlusion, Myocardial Infarction (Heart Attack), Peripheral Arterial Occlusive Diseases, and Others), Distribution Channel (Hospital Pharmacies, Retail and Drug Stores, Direct Sales (API and FDF), and Online Pharmacies), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Oct 2025
- Report Code : TIPRE00041040
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 390
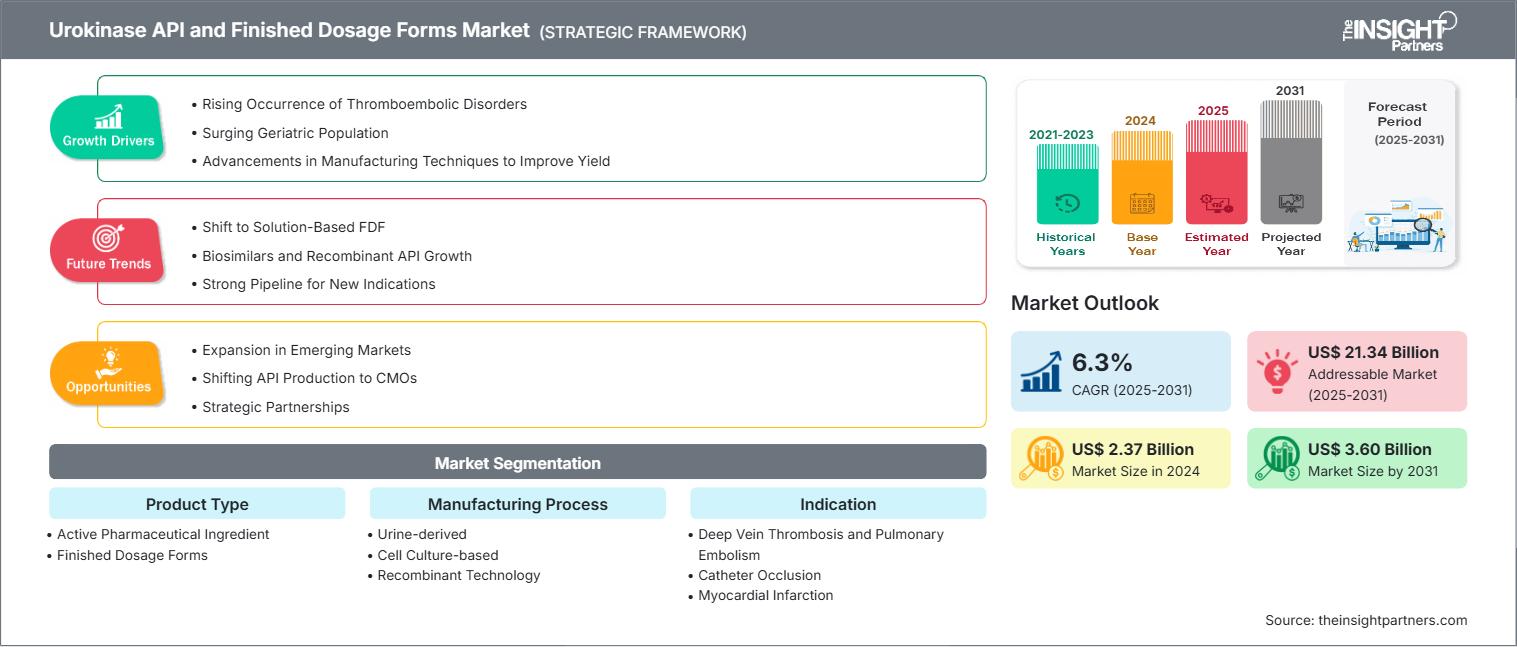
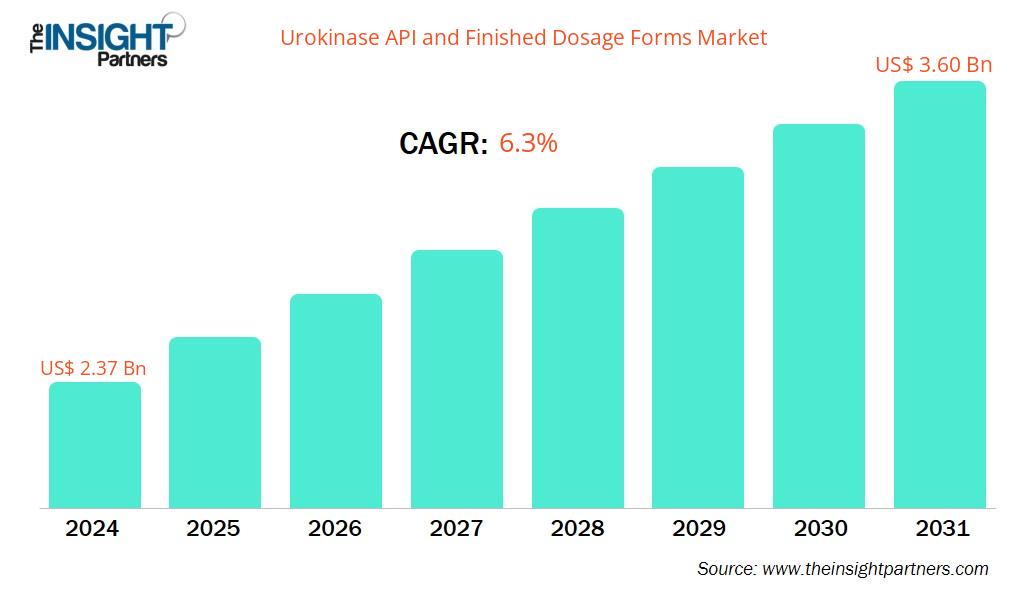
The Urokinase API and Finished Dosage Forms Market size is projected to reach US$ 3.60 billion by 2031 from US$ 2.37 billion in 2024. The market is expected to register a CAGR of 6.3% during 2025–2031.
Urokinase API and Finished Dosage Forms Market Analysis
The market is witnessing steady expansion, with an increasing incidence of cardiovascular and thrombotic disorders such as deep vein thrombosis, pulmonary embolism, myocardial infarction, and ischemic stroke. In addition, favorable regulatory trends such as faster approvals or reintroducing urokinase products for new indications contribute to the market growth.
Urokinase API and Finished Dosage Forms Market Overview
Increased incidence of cardiovascular and thrombotic ailments worldwide, notably in elderly populations, is a positive factor for the market. Competitive players focus on regulatory clearance, geographical expansion, and formulation changes to capture the market share. However, stringent regulatory requirements and the availability of alternate treatment therapies limit market expansion.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONUrokinase API and Finished Dosage Forms Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Urokinase API and Finished Dosage Forms Market Drivers and Opportunities
Market Drivers:
- Rising Occurrence of Thromboembolic Disorders: The increasing incidence of thromboembolic disorders drives the demand for treatment, chiefly urokinase, as an active pharmaceutical ingredient, as well as in finished dosage form.
- Growing Proportion of Geriatric Population: Increasing life expectancy, paralleled by a decline in birth rates, leads to an increasing elderly population. This demographic is prone to thromboembolism due to immobility, co-morbid illness, and physiological changes. . The increasing prevalence of thrombotic disorders in the elderly and the changing healthcare landscape aimed at geriatric care provide all grounds for a continually growing market.
- Advancements in Manufacturing Techniques to Boost Production: High-throughput techniques, involving genetically engineered cell lines, have increased productivity at the cellular level. The synthesis, regulation, and secretion processes have been optimized, resulting in increased urokinase production.
Market Opportunities:
- Emerging Markets Expansion: Greater access to the increased investment and growing healthcare awareness in Asia Pacific, Latin America, and the Middle East provides an untapped potential for penetrating markets and increasing sales.
- Shifting API Production to CMOs: Collaborating with biotech companies and contract manufacturing organizations (CMOs), pharmaceutical companies facilitate using advanced bioprocessing abilities, decrease production costs, and comply with regulations. Outsourcing to CMOs enables them to take advantage of expertise, state-of-the-art biotechnological processes, and capacity infrastructure with minimal capital investment plus operational complexities.
- Strategic Partnerships: The competitive landscape is transforming with strategic agreements making room for innovations, meeting the growing need for pulmonary embolism and DVT thrombolytic therapies. Pharmaceutical companies are increasingly entering into alliances, licensing agreements, and collaborative partnerships to share technological resources and reduce R&D costs. These strategic moves enable them to capitalize on market growth fully.
Urokinase API and Finished Dosage Forms Market Report Segmentation Analysis
The urokinase API and finished dosage forms market is divided into different segments to give a clearer view of how it works, its growth potential, and the latest trends. Below is the standard segmentation approach used in industry reports:
By Product Type:
- Active Pharmaceutical Ingredient: The API is the component of urokinase that is biochemically active for thrombolysis. It is produced through biological processes, such as cell cultures or recombinant products, and then formulated into dosage forms.
- Finished Dosage Forms: The finished drug products of urokinase are produced with a pre-formulated API packed and labeled in dosage forms for patients, typically for treating pulmonary embolism and/or occlusions resulting from catheters.
By Manufacturing Process:
- Urine-derived
- Cell Culture-based
- Recombinant Technology
By Indication:
- Deep Vein Thrombosis and Pulmonary Embolism
- Catheter Occlusion
- Myocardial Infarction (Heart Attack)
- Peripheral Arterial Occlusive Diseases
- Others
By Distribution Channel:
- Hospital Pharmacies
- Retail and Drug Stores
- Direct Sales (API and FDF)
- Online Pharmacies
By Geography:
- North America
- Europe
- Asia Pacific
- South & Central America
- Middle East & Africa
Urokinase API and Finished Dosage Forms
Urokinase API and Finished Dosage Forms Market Regional InsightsThe regional trends influencing the Urokinase API and Finished Dosage Forms Market have been analyzed across key geographies.
Urokinase API and Finished Dosage Forms Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 2.37 Billion |
| Market Size by 2031 | US$ 3.60 Billion |
| Global CAGR (2025 - 2031) | 6.3% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Urokinase API and Finished Dosage Forms Market Players Density: Understanding Its Impact on Business Dynamics
The Urokinase API and Finished Dosage Forms Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Urokinase API and Finished Dosage Forms Market Share Analysis by Geography
The urokinase API and finished dosage forms market in Asia Pacific is witnessing the fastest growth. The rising burden of thromboembolism and CVDs, including DVT, PE, and myocardial infarction, along with aging, urbanization, obesity, and diabetes, are key factors driving market growth. Emerging markets in South America, the Middle East, and Africa have untapped opportunities for urokinase API and finished dosage forms providers to expand.
The growth of the urokinase API and finished dosage forms market varies across regions due to factors such as growing healthcare infrastructure in the APAC region and technological and product innovations. Below is a summary of market share and trends by region:
1. North America
- Market Share: Holds a significant portion of the global market
-
Key Drivers:
- North America's sophisticated healthcare system and high healthcare spending lead to improved access to new therapies and specialized care. Leading organizations to drive pharmaceutical R&D focused on developing and commercializing urokinase-based products.
- Trends: Increased urokinase utilization in acute care areas for thrombolytic care.
2. Europe
- Market Share: Substantial market share
-
Key Drivers:
- Europe has an aging population, who have a higher risk of thrombotic diseases. Increasing incidence of these conditions creates demand for the appropriate use of thrombolytic agents, such as urokinase.
- Trends: Focus on clinic‑based care and earlier intervention.
3. Asia Pacific
- Market Share: Fastest-growing region with a rising market share every year
-
Key Drivers:
- Governments in the region are investing in hospital infrastructure, emergency services, clinics, and increasing healthcare funding and awareness amongst physicians and patients for thrombolytics.
- Trends: Growth of biosimilar formulations.
4. South and Central America
- Market Share: Growing market with steady progress
-
Key Drivers:
- Cardiovascular and thrombotic diseases place a significant burden on Latin America. Growing awareness campaigns are improving recognition and diagnosis, driving demand for urokinase.
- Trends: Brazil and Argentina are undertaking public health awareness programs.
5. Middle East and Africa
- Market Share: Although small, it is growing quickly
-
Key Drivers:
- Countries in the Middle East are investing in healthcare infrastructure with increased hospital capacity and emergency care, and upgrading facilities. This investment creates capacity for advanced treatments such as urokinase.
- Trends: Opportunities in under-penetrated areas & increasing awareness.
Urokinase API and Finished Dosage Forms Market Players Density: Understanding Its Impact on Business Dynamics
High Market Density and Competition
Competition is strong due to the presence of established players such as Taj Pharmaceuticals Ltd and Jiangxi Haoran Bio-Pharma Co., Ltd. Regional and niche providers add to the competitive landscape across regions.
The high level of competition urges companies to stand out by offering:
- Advanced Products
- Product Approvals
- Compliance with Regulatory Guidelines
Opportunities and Strategic Moves
- Transitioning from urine-derived or pre-recombinant expression systems toward recombinant expression systems allows higher purity, consistency, and lower immunogenicity. Biosimilars are adopted rapidly, especially in cost-sensitive markets.
- Beyond traditional uses in DVT and PE, urokinase is explored for maintaining catheter patency and intrapleural fibrinolytic therapy. Additionally, there is growing potential for its use in stroke management, including ischemic stroke protocols, peripheral vascular occlusions, and combination therapies.
Other companies analyzed during the course of research:
- Chandra Bhagat Pharma
- ARISTO Pharmaceuticals Private Limited
- medac GmbH.
- Dabur India Ltd
- Samarth Life Sciences Pvt. Ltd.
- Kraeber & Co GmbH
- TTK Healthcare Limited
- Life Medicare & Biotech Private Limited
- BBT Biotech GmbH
- Unichem Laboratories Ltd.
Urokinase API and Finished Dosage Forms Market News and Recent Developments
- Sequel Pharma Executes Agreement with CDMO for Drug Product Manufacturing. Microbix Biosystems Inc. announced that its funding and commercialization partner, Sequel Pharma, LLC, has signed an agreement with an international contract development and manufacturing organization (CDMO) for the production, formulation, and packaging of the Kinlytic urokinase drug.
- HAS Healthcare Advanced Synthesis SA completes the acquisition of Cerbios-Pharma SA to establish a top-tier global group in the CDMO industry. This strategic transaction marks a significant milestone for the two companies and sets the stage for creating a leading global solutions provider in the CDMO industry.
Urokinase API and Finished Dosage Forms Market Report Coverage and Deliverables
The "Urokinase API and Finished Dosage Forms Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Urokinase API and Finished Dosage Forms Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Urokinase API and Finished Dosage Forms Market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Urokinase API and Finished Dosage Forms Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the Urokinase API and Finished Dosage Forms Market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





 Get Free Sample For
Get Free Sample For