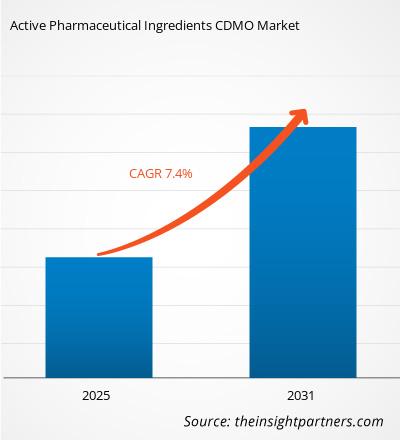
The Active Pharmaceutical Ingredients CDMO Market is expected to register a CAGR of 7.4% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Synthesis (Synthetic, and Biotech). The report further presents analysis based on the Drug (Innovative, and Generics), Workflow(Clinical, Commercial). The global analysis is further broken-down at regional level and major countries. The Report Offers the Value in USD for the above analysis and segments.
Purpose of the Report
The report Active Pharmaceutical Ingredients CDMO Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Active Pharmaceutical Ingredients CDMO Market SegmentationDrug - Innovative
- Generics
Workflow - Clinical
- Commercial
Synthesis - Synthetic
- Biotech
- Innovative
- Generics
Workflow - Clinical
- Commercial
Synthesis - Synthetic
- Biotech
- Synthetic
- Biotech
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Active Pharmaceutical Ingredients CDMO Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Active Pharmaceutical Ingredients CDMO Market Growth Drivers- Higher demand for generic drugs: The API CDMO market is being driven by expiring patents of drugs, a growing demand for generic drugs in the global market. Generic drugs require the high-volume production of APIs at competitive costs. Therefore, CDMOs offer cost-effective solutions for such generic drug manufacturers as regards API synthesis, purification, and regulatory compliance, thereby driving the growth of the market.
- Growing Biologics and Biosimilars: Biologics and biosimilars have exponentially growing demand, and both hold the potential promise to tackle chronic and complex diseases. The specialized skills needed to manufacture APIs for biologics make CDMOs a critical growth area for API manufacturing companies. Today, pharmaceutical companies are showing an increased tendency to partner up with API CDMOs who can help them carry on such complex processes.
- Emphasis on Cost Efficiency and Outsourcing:Cost Efficiency and Outsourcing Emphasis API production outsourced by pharmaceutical firms reduces overhead costs, facilitates operations, and directs core activities on drug discovery and marketing. CDMOs provide expertise in manufacturing skills that allow drug developers to achieve the cost and time requirements they need and thereby help maintain the supply of APIs into the market that fuels the demand for these services.
Active Pharmaceutical Ingredients CDMO Market Future Trends- Increased Emphasis on Quality and Regulatory Compliance: The pharmaceutical industry is undergoing a massive trend concerning the quality standards and regulation with increased regulation. API CDMOs are investing in robust quality control and assurance systems to meet the stringent global standards set by regulatory bodies like the FDA and EMA, thereby satisfying both regulatory authorities and end consumers.
- Advancements in Continuous Manufacturing: Continuous manufacturing is gaining ground in the API CDMO market as it has several benefits over traditional batch manufacturing, for example, increased efficiency and scalability, as well as lower production costs. Continuous integration of processes into API production gives greater flexibility to production and enhances product consistency, thus better meeting the requirements of growing demand by utilizing more resources.
- Green and Sustainable Manufacturing Practices: Sustainability has emerged as a key focus for the API CDMO market. Companies are adopting green manufacturing technologies to minimize their footprint in the environment. These comprise energy-efficient technologies, reduced waste, and use of green chemistry, all consistent with the growing need for production of drugs with eco-friendly methodologies, leading to long-term operational efficiencies.
Active Pharmaceutical Ingredients CDMO Market Opportunities- Emerging markets: Emerging markets, especially in the Asia-Pacific, are significant growth areas for API CDMOs, given growing access to health care, an increasing demand for affordable generics, and developing local pharmaceutical industries. Here, with these manufacturing facilities in place, the CDMOs can ride the wave of growing demand for generic as well as innovative drugs.
- Strategic partnerships with pharma companies: API CDMOs can collaborated with pharmaceutical companies, especially smaller biotech companies that do not have large-scale manufacturing capabilities. Such alliances will allow CDMOs to expand their client base, increase their production capacity, and provide integrated services, from API development to final drug product manufacturing.
- Investment in Biotechnology and Novel Drug Development: With biotechnology increasingly important and with the entry of new biologic and biosimilar drugs into the market, the API CDMOs can invest in specialized manufacturing capabilities for such complex drug types. Related to the production of high-value biologic APIs, there are significant growth opportunities for CDMOs because the demand for biologics is increasing all over the world.
- Increased Emphasis on Quality and Regulatory Compliance: The pharmaceutical industry is undergoing a massive trend concerning the quality standards and regulation with increased regulation. API CDMOs are investing in robust quality control and assurance systems to meet the stringent global standards set by regulatory bodies like the FDA and EMA, thereby satisfying both regulatory authorities and end consumers.
- Advancements in Continuous Manufacturing: Continuous manufacturing is gaining ground in the API CDMO market as it has several benefits over traditional batch manufacturing, for example, increased efficiency and scalability, as well as lower production costs. Continuous integration of processes into API production gives greater flexibility to production and enhances product consistency, thus better meeting the requirements of growing demand by utilizing more resources.
- Green and Sustainable Manufacturing Practices: Sustainability has emerged as a key focus for the API CDMO market. Companies are adopting green manufacturing technologies to minimize their footprint in the environment. These comprise energy-efficient technologies, reduced waste, and use of green chemistry, all consistent with the growing need for production of drugs with eco-friendly methodologies, leading to long-term operational efficiencies.
Active Pharmaceutical Ingredients CDMO Market Opportunities- Emerging markets: Emerging markets, especially in the Asia-Pacific, are significant growth areas for API CDMOs, given growing access to health care, an increasing demand for affordable generics, and developing local pharmaceutical industries. Here, with these manufacturing facilities in place, the CDMOs can ride the wave of growing demand for generic as well as innovative drugs.
- Strategic partnerships with pharma companies: API CDMOs can collaborated with pharmaceutical companies, especially smaller biotech companies that do not have large-scale manufacturing capabilities. Such alliances will allow CDMOs to expand their client base, increase their production capacity, and provide integrated services, from API development to final drug product manufacturing.
- Investment in Biotechnology and Novel Drug Development: With biotechnology increasingly important and with the entry of new biologic and biosimilar drugs into the market, the API CDMOs can invest in specialized manufacturing capabilities for such complex drug types. Related to the production of high-value biologic APIs, there are significant growth opportunities for CDMOs because the demand for biologics is increasing all over the world.
Active Pharmaceutical Ingredients CDMO Market Regional Insights
The regional trends and factors influencing the Active Pharmaceutical Ingredients CDMO Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Active Pharmaceutical Ingredients CDMO Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Active Pharmaceutical Ingredients CDMO Market
Active Pharmaceutical Ingredients CDMO Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 7.4% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Drug
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Active Pharmaceutical Ingredients CDMO Market Players Density: Understanding Its Impact on Business Dynamics
The Active Pharmaceutical Ingredients CDMO Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Active Pharmaceutical Ingredients CDMO Market are:
- Boehringer Ingelheim International GmbH
- Cambrex Corporation
- CordenPharma International
- Lonza Group
- WuXi AppTec
- Samsung Biologics
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Active Pharmaceutical Ingredients CDMO Market top key players overview
Key Selling Points- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Active Pharmaceutical Ingredients CDMO Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Active Pharmaceutical Ingredients CDMO Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
Frequently Asked Questions
What is driving the growth of the API CDMO market?
The growth is driven by the increasing demand for generic drugs, biologics, and biosimilars, and the growing focus on cost efficiency and outsourcing by pharmaceutical companies.
How are regulatory requirements influencing the API CDMO market?
Global trend toward more focus on quality and compliance due to requirements from regulatory authorities; these CDMOs have heavy investments in quality control systems, regulatory compliance, in a broad sense, for conformity of all their products with FDA/European Medicines Agency EMA standards of stringent safety and efficacy guidelines of pharmaceutical products.
What role does continuous manufacturing play in the API CDMO market?
Continuous manufacturing plays a very crucial role in increasing production efficiency, scalability, and cost-effectiveness. The trend of continuous manufacturing allows API CDMOs to meet growing market demands with improved product consistency and reduced production times
What opportunities exist for API CDMOs in emerging markets?
API CDMOs, especially in Asia-Pacific, have great growth opportunities. With growing demands for affordable generics, expanding access to healthcare, and local pharmaceutical industries on the rise, this becomes a great opportunity for CDMOs to establish their operations and capture local market growth
Which region is expected to witness the fastest growth rate by 2031?
Asia-Pacific region is likely to witness the fastest growth rate during the forecast period.
What are the benefits API CDMOs can gain through alliances with pharmaceutical companies?
One benefit of forming strategic partnerships with pharmaceutical companies, particularly smaller biotech firms, to APIs CDMOs, would be to enhance their services by tapping into new markets and grow client base.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends
Yes! We provide a free sample of the report, which includes Report Scope (Table of Contents), report structure, and selected insights to help you assess the value of the full report. Please click on the "Download Sample" button or contact us to receive your copy.
Absolutely — analyst assistance is part of the package. You can connect with our analyst post-purchase to clarify report insights, methodology or discuss how the findings apply to your business needs.
Once your order is successfully placed, you will receive a confirmation email along with your invoice.
• For published reports: You’ll receive access to the report within 4–6 working hours via a secured email sent to your email.
• For upcoming reports: Your order will be recorded as a pre-booking. Our team will share the estimated release date and keep you informed of any updates. As soon as the report is published, it will be delivered to your registered email.
We offer customization options to align the report with your specific objectives. Whether you need deeper insights into a particular region, industry segment, competitor analysis, or data cut, our research team can tailor the report accordingly. Please share your requirements with us, and we’ll be happy to provide a customized proposal or scope.
The report is available in either PDF format or as an Excel dataset, depending on the license you choose.
The PDF version provides the full analysis and visuals in a ready-to-read format. The Excel dataset includes all underlying data tables for easy manipulation and further analysis.
Please review the license options at checkout or contact us to confirm which formats are included with your purchase.
Our payment process is fully secure and PCI-DSS compliant.
We use trusted and encrypted payment gateways to ensure that all transactions are protected with industry-standard SSL encryption. Your payment details are never stored on our servers and are handled securely by certified third-party processors.
You can make your purchase with confidence, knowing your personal and financial information is safe with us.
Yes, we do offer special pricing for bulk purchases.
If you're interested in purchasing multiple reports, we’re happy to provide a customized bundle offer or volume-based discount tailored to your needs. Please contact our sales team with the list of reports you’re considering, and we’ll share a personalized quote.
Yes, absolutely.
Our team is available to help you make an informed decision. Whether you have questions about the report’s scope, methodology, customization options, or which license suits you best, we’re here to assist. Please reach out to us at sales@theinsightpartners.com, and one of our representatives will get in touch promptly.
Yes, a billing invoice will be automatically generated and sent to your registered email upon successful completion of your purchase.
If you need the invoice in a specific format or require additional details (such as company name, GST, or VAT information), feel free to contact us, and we’ll be happy to assist.
Yes, certainly.
If you encounter any difficulties accessing or receiving your report, our support team is ready to assist you. Simply reach out to us via email or live chat with your order information, and we’ll ensure the issue is resolved quickly so you can access your report without interruption.















- Boehringer Ingelheim International GmbH
- Cambrex Corporation
- CordenPharma International
- Lonza Group
- WuXi AppTec
- Samsung Biologics
- Fujifilm Diosynth Biotechnologies
- Patheon (Thermo Fisher Scientific)
- Siegfried Holding AG
- Aenova Group






 Get Free Sample For
Get Free Sample For