Infectious Disease In vitro Diagnostics Market Size and Growth 2031
Infectious Disease In vitro Diagnostics Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: Application (HIV or AIDS, Tuberculosis, Hepatitis B and C, Malaria, and Others), End User (Hospitals and Clinics, Diagnostic Laboratories, Blood Bank, and Others), and Geography
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Jul 2025
- Report Code : TIPRE00040978
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 150
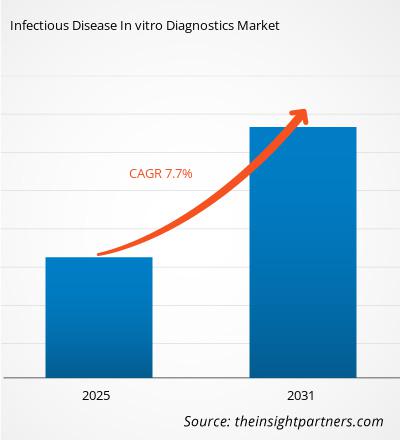
The infectious disease in vitro diagnostics market size is projected to reach US$ 76.71 billion by 2031 from US$ 45.69 billion in 2024. The market is expected to register a CAGR of 7.7% during 2025–2031. Technological advancements are likely to bring new trends in the infectious disease in vitro diagnostics market.
Infectious Disease In vitro Diagnostics Market Analysis
The infectious disease in vitro diagnostics market is witnessing substantial growth due to the rising global burden of infectious diseases—including HIV, tuberculosis, COVID‑19, and emerging zoonotic threats—which has intensified demand for early, accurate detection. Technological innovation also plays a pivotal role: advances in molecular diagnostics such as PCR, next-generation sequencing, microfluidics, and AI-enhanced platforms are enabling faster, more precise, and multiplex testing capabilities. Another major growth driver is the expansion of point‑of‑care and home testing, bolstered by telemedicine and consumer preference for rapid, at‑home diagnostics. Demographic trends, notably an aging global population susceptible to infections, further support market expansion.
Infectious Disease In vitro Diagnostics Market Overview
The global infectious disease in vitro diagnostics market is accelerating owing to the aging global population that magnifies this need, as immunosenescence increases susceptibility to infections, thereby fueling demand for reliable diagnostic tools. A mounting burden of infectious diseases—including long‑standing threats like HIV, tuberculosis, malaria, and emerging ones such as COVID‑19, Ebola, and Zika—is sustaining demand for rapid, accurate diagnostics. Rapid technological innovation is a critical catalyst. Next‑generation molecular diagnostics—PCR, NAAT, and NGS—along with advanced immunoassays, lab-on-a-chip systems, and point-of-care devices are boosting sensitivity, specificity, and speed. Integration of AI, machine learning, and automation further enhances diagnostic accuracy and efficiency, streamlining workflows and enabling predictive surveillance. Together, these factors are synergistically propelling the global market forward.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONInfectious Disease In vitro Diagnostics Market: Strategic Insights

-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Infectious Disease In vitro Diagnostics Market Drivers and Opportunities
Increasing Prevalence of Infectious Diseases Bolsters Market
As per the World Health Organization (WHO), tuberculosis (TB) affects 10 million people globally each year. Even though TB can be both prevented and cured, it still claims 1.5 million lives annually, making it among the leading infectious causes of death worldwide. The majority of TB cases occur in low- and middle-income countries, though the disease is found across the globe. Nearly half of all TB cases are recorded in the following eight countries: Bangladesh, China, India, Indonesia, Nigeria, Pakistan, the Philippines, and South Africa.
In alignment with global health trends, the prevalence of HIV continues to escalate worldwide. According to data from the World Health Organization, an estimated 39.9 million individuals were living with HIV as of the end of 2023, with projections ranging from 36.1 to 44.6 million. Approximately 0.6% of the global adult population aged 15–49 years is affected, although the magnitude and implications of the epidemic vary substantially across different countries and regions.
Furthermore, increasing fungal infections are one of the major concerns among a large population base globally, affecting millions. According to the Global Action Fund for Fungal Infections (GAFFI) 2024, more than 80 million people are at high risk of developing fungal disease every year, and approximately 6.55 million patients develop life-threatening fungal infections, of which 3.75 million people die annually. Thus, the growing cases of infectious diseases create a significant need for in vitro diagnosis to detect diseases and infections before proceeding with the desired treatment. Further, the escalating prevalence of HIV, hepatitis, tuberculosis, influenza, and new viral infections has intensified the demand for rapid and precise diagnostic solutions. Innovations in molecular diagnostics, better point-of-care testing, and greater emphasis on early detection and disease surveillance are collectively driving the adoption of in vitro diagnostic methodologies across both developed and emerging healthcare markets.
Government Initiatives to Increase Awareness Regarding Infectious Diseases to Create Growth Opportunities
Government efforts to raise awareness on infectious diseases, as well as their diagnosis, hold a major opportunity in the infectious disease in vitro diagnostics market. Initiatives focused on early detection, disease management, and strengthening the health system are fueling the demand for diagnostic products worldwide.
Public health initiatives such as immunization campaigns, school-based education programs, and community-level screenings are raising public awareness and utilization of diagnostic tests. Disease-control national programs for diseases such as tuberculosis, HIV, hepatitis, malaria, and dengue incorporate a mass screening element into the program, leading to sustained demand for centralized and point-of-care diagnostic platforms.
Governments are also backing diagnostic innovation with R&D investments, regulatory expediting, and public-private collaborations. The US NIH's RADx program and equivalents in the EU and Asia have intensified the development and deployment process of rapid and molecular tests. In lower- and middle-income economies, WHO's Essential Diagnostics List (EDL) and programs such as FIND and the Global Fund are supporting high-burden disease diagnostics priority setting and funding. Also, through the Nigerian Center for Disease Control (NCDC) and the Ministry of Health, the government promotes public health campaigns targeting malaria, HIV/AIDS, and tuberculosis. These campaigns emphasize early detection, prevention, and management, encouraging more people to seek diagnostic testing. As awareness grows, healthcare providers and diagnostic centers increasingly adopt advanced IVD technologies to meet the rising demand for reliable and rapid tests. Nationwide HIV screening programs supported by government initiatives have increased the need for efficient in vitro diagnostic kits. Similarly, in 2023, under the National Health Mission, the Indian government launched the Free Diagnostics Service Initiative (FDSI) to provide accessible and affordable diagnostics, reducing out-of-pocket expenses on healthcare. Further, in December 2024, Mexico's Ministry of Health's National Institute of Respiratory Diseases (INER) "Ismael Cosío Villegas" launched its first infectious disease diagnostic equipment, based on T2 Magnetic Resonance (T2MR) technology. The technology will help combat antimicrobial resistance (AMR) and speed up diagnosis.
Additionally, greater investment in national preparedness strategies and disease surveillance, particularly after the COVID-19 pandemic, has placed infectious disease diagnosis at the forefront. These efforts not only increase testing infrastructure but also create favorable regulatory and funding conditions, setting government-led awareness campaigns as a primary opportunity for growth within the IVD market.
Partnerships with international health organizations and government subsidies help improve access to these diagnostic tools, especially in rural and underserved areas. This supportive environment attracts investments from diagnostic companies aiming to develop and distribute innovative testing solutions tailored to the needs of a specific country or region. Overall, government-driven awareness campaigns are expected to create lucrative opportunities in the infectious disease IVD market by creating sustained demand for diagnostic innovations.
Infectious Disease In vitro Diagnostics Market Report Segmentation Analysis
Key segments that are the foundation of the infectious disease in vitro diagnostics market analysis are application and end user.
- Based on application, the infectious disease in vitro diagnostics market is segmented into HIV/AIDS, Tuberculosis, Hepatitis B & C, Malaria, and Others. The Hepatitis B & C segment held the largest share of the market in 2024.
- In terms of end user, the infectious disease in vitro diagnostics market is categorized into hospitals and clinics, diagnostics laboratories, blood bank, and others. The hospitals and clinics segment dominated the market in 2024.
Infectious Disease In vitro Diagnostics Market Share Analysis by Geography
The geographical scope of the infectious disease in vitro diagnostics market report is divided into five regions: North America, Asia Pacific, Europe, the Middle East and Africa, and South and Central America.
North America held a significant share of the market in 2024. The increasing acceptance of technologically advanced products, rising research and development activities, and growing use of in vitro diagnostics kits for infectious disease diagnosis, as well as the presence of large healthcare businesses, are among the key factors propelling the growth of the infectious diseases in vitro diagnostics market in North America. The region is experiencing an increasing prevalence of infectious diseases and multiple product launches for its diagnosis by key players. Additionally, growing awareness among patients and healthcare providers about the importance of early disease detection supports market expansion. Government initiatives and funding aimed at strengthening disease surveillance and preparedness further bolster the sector. The presence of leading diagnostic companies and favorable regulatory frameworks from agencies like the FDA facilitates the quick approval and commercialization of new tests. Moreover, an aging population, which is more vulnerable to infections, is increasing the need for regular diagnostic screening.
Infectious Disease In vitro Diagnostics
Infectious Disease In vitro Diagnostics Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 45.69 Billion |
| Market Size by 2031 | US$ 76.71 Billion |
| Global CAGR (2025 - 2031) | 7.7% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Application
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Infectious Disease In vitro Diagnostics Market Players Density: Understanding Its Impact on Business Dynamics
The Infectious Disease In vitro Diagnostics Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Infectious Disease In vitro Diagnostics Market News and Recent Developments
The infectious disease in vitro diagnostics market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. A key development in the market is listed below:
- bioMérieux, a world leader in the field of in vitro diagnostics, announced the launch of its WATCHFIRE molecular testing solution. The WATCHFIRE Respiratory (R) Panel, targeting 22 pathogens, will run on the BIOFIRE FILMARRAY TORCH instrument, integrated with FIREWORKS software, to deliver real-time trending of viruses and bacteria present in wastewater samples. (Source: bioMerieux SA, Press Release, April 2025)
- Roche received FDA approval for the first molecular test to screen for malaria in blood donors in the US. Roche's cobas Malaria test was approved by the FDA for use on the cobas 6800/8800 Systems. The test identifies infected blood units, making the blood supply safer. The test screens for the five main species of Plasmodium parasites that cause human infection. It can be used to screen blood, organ, and tissue donors, improving blood safety and availability. (Source: F. Hoffmann-La Roche Ltd, Press Release, March 2024)
Infectious Disease In vitro Diagnostics Market Report Coverage and Deliverables
The "Infectious Disease In vitro Diagnostics Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Infectious disease in vitro diagnostics market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Infectious disease in vitro diagnostics market trends and market dynamics, such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Infectious disease in vitro diagnostics market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments in the infectious disease in vitro diagnostics market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





 Get Free Sample For
Get Free Sample For