The medical device and diagnostics contract research organization market in APAC is expected to grow from US$ 2,394.72 million in 2021 to US$ 4,110.91 million by 2028; it is estimated to grow at a CAGR of 8.0% from 2021 to 2028.
Australia, China, India, Japan, and South Korea are major economies in APAC. Market consolidation on rise is the major factor driving the growth of the APAC medical device and diagnostics contract research organization market. The CRO services industry is highly fragmented, with several hundred small and medium-sized limited-service providers, and a small number of large and full-service CROs. There are a few barriers for smaller CROs to enter the APAC market. However, the full-service CROs require efficient infrastructure with an ability to simultaneously manage multiple complex testing services across numerous geographies, establish the requisite relationships with strategic partners, and develop relevant therapeutic and expertise to meet the needs of end users. Over the past a few years, the consolidation across the industry is an emerging trend followed by most of the prime players to strengthen their service offerings and garner the major market share in the APAC medical device & diagnostics contract research organization market. For instance, PAREXEL International Corp. acquired The Medical Affairs Company in 2017. This has led to the generation of larger CROs with the wide geographic diversification, extensive therapeutic and development expertise, enormous capital and technical resources to manage the demanding drug development programs, medical device designing and research as well as management of regulatory affairs for pharmaceutical and biopharmaceutical companies.
Countries in APAC are facing an increasing incidence of COVID-19. Accompanying the catastrophic loss of life that the virus has caused is the impact on the global economy, which has reeled from the effects of the pandemic. The COVID-19 pandemic has critically impacted the region; however, it has also provided an opportunity for stakeholders to realign their future path based on strategic intent and focus. COVID-19 has created an extraordinary emergency that is particularly affecting the supply chain. The supply chain disruptions, along with the enormous need for medical devices and protective health care material, have generated the need for new initiatives and the use of emerging technologies such as three-dimensional printing (3DP) to come forward and support health care professionals and supply chain. The COVID-19 pandemic has adversely impacted the region, but it has also provided an opportunity for stakeholders to realign their future path based on strategic intent and focus. India and China are the most preferred destinations for outsourcing, where a large pool of skilled labor is available at a lower cost. With increasing pressure to reduce manufacturing costs and accelerate time-to-market, many devices and diagnostics companies are looking to conduct clinical trial operations in APAC. Increasingly, third-party outsourcing service providers are playing a greater role in supporting and managing the delivery of key business functions like medical device development, sales and marketing, and regulatory compliance services in APAC.
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the APAC medical device and diagnostics contract research organization market. The APAC medical device and diagnostics contract research organization market is expected to grow at a good CAGR during the forecast period.
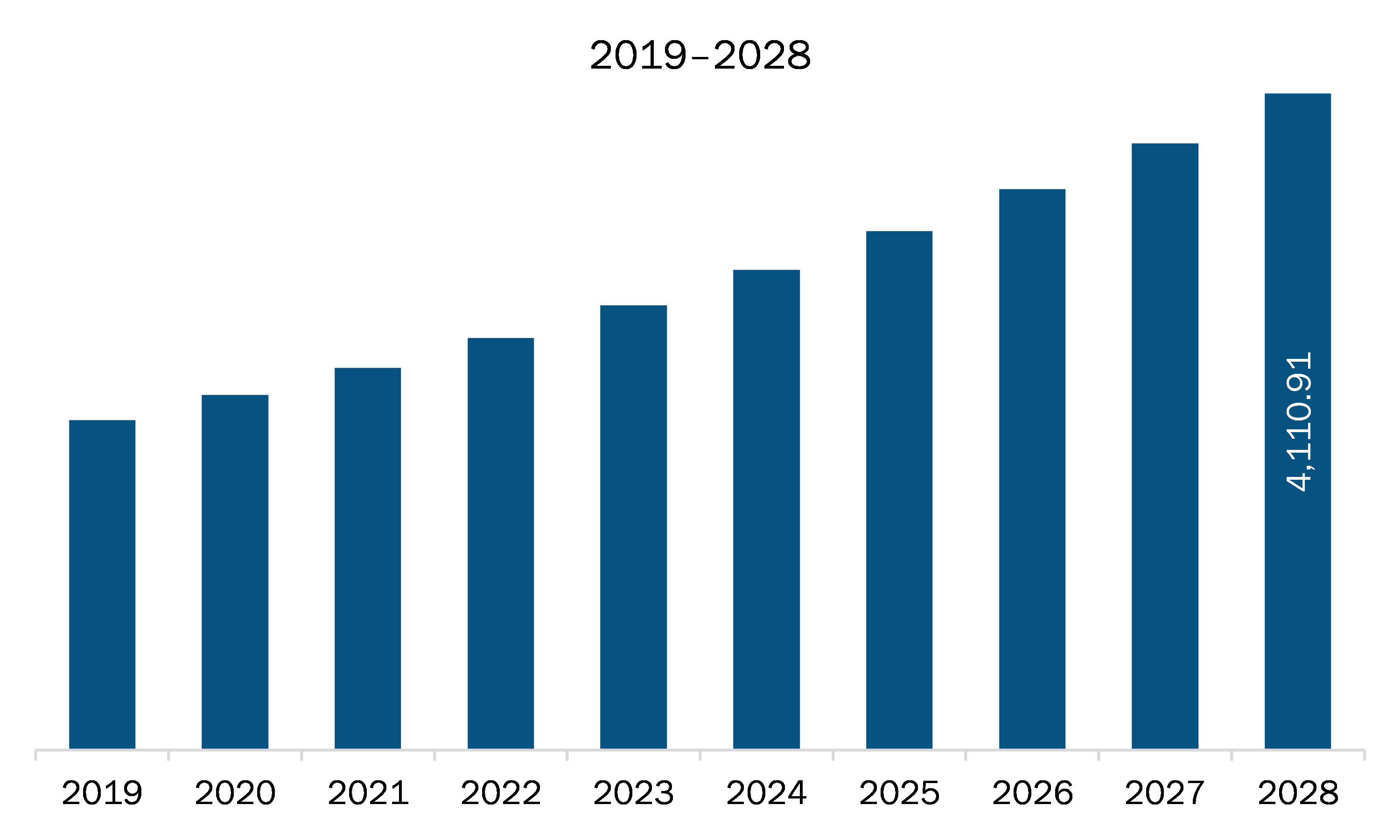
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
APAC Medical Device and Diagnostics Contract Research Organization Market Segmentation
APAC Medical Device and Diagnostics Contract Research Organization Market – By Type
- Medical Devices
- Diagnostics
- Cardiac Biomarkers
- Diabetes Management
- Oncology
- Infectious Diseases
- Hematology
- Chemistry and Immunoassays
- Molecular Diagnostics
- Others
APAC Medical Device and Diagnostics Contract Research Organization Market – By Services
- Clinical Data Management
- Monitoring
- Clinical Project Management
- Medical Writing
- Clinical Auditing
- Digital Health
- Clinical Strategy
- Others
APAC Medical Device and Diagnostics Contract Research Organization Market, by Country
- Australia
- China
- India
- Japan
- South Korea
- Rest of APAC
APAC Medical Device and Diagnostics Contract Research Organization Market - Companies Mentioned
- Charles River Laboratories, Inc
- ICON PLC
- IQVIA
- Laboratory Corporation of America Holdings
- North American Science Associates, Inc.
- PAREXEL International Corporation
- Qserve Group B.V.
- WUXI APPTEC
Asia Pacific Medical Device and Diagnostics Contract Research Organization Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 2,394.72 Million |
| Market Size by 2028 | US$ 4,110.91 Million |
| CAGR (2021 - 2028) | 8.0% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Type
|
| Regions and Countries Covered |
Asia-Pacific
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends







 Get Free Sample For
Get Free Sample For