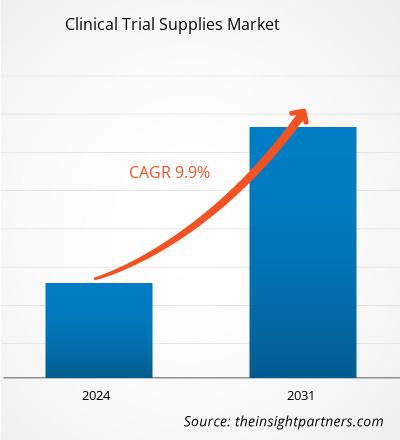
The Clinical Trial Supplies Market size is projected to reach US$ 8085.87 million by 2031 from US$ 3819.36 million in 2023. The market is expected to register a CAGR of 9.9% in 2024–2031. With the increasing costs of drug discovery, clinical trial supplies are obtaining more importance. In addition, implementing more stringent handling requirements for a type of biopharmaceutical product starting clinical trials and clinical trial supplies strategy needs to be continuously improved. The growth of the global clinical trials supplies market is attributed to key driving factors such as increasing R&D expenditures in the pharmaceutical & biopharmaceutical companies, increase in the number of clinical trials, and higher costs of drug development in developed nations. However, the rising cost of drug development and clinical trials and challenges for clinical trials due to the coronavirus's negative impact are expected to restrain the market growth during the forecast years. The significantly growing chronic diseases across the world are likely to remain a clinical trial supplies market trend.
Clinical Trial Supplies Market Analysis
The growing healthcare sector in developing countries of Asia-Pacific creates better opportunities for the clinical trial supplies management market players to expand their business. The huge patient population in these countries is generating demand for more clinical trials. More clinical trials are conducted in the Asia Pacific than in the US or Europe. This shift is ascribed to low operational costs, large patient recruitment potential, contract research organization's growth, favorable regulatory environment, and better clinical trial capacity and quality. In developed regions such as North America and Europe, ~35% of trials are delayed due to problems in patient recruitment, and one-fifth of the trials are refrained from enrolment due to insufficient subjects. As per the report “Clinical Trials in Asia: A World Health Organization database study,” from 2008 to 2017, the average yearly rise in the number of clinical trials conducted was 41.9% in Iran, 27.1% in Sri Lanka, 23.3% in China, 21.3% in India, 18.4% in Japan, 14.7% in Thailand, 8.4% in Malaysia, and 12.9% in Korea.
The rising number of clinical trial supply market players is expected to drive the global clinical trial supplies market.
Clinical Trial Supplies Market Overview
The clinical trial is an investigation study that defines whether a medical approach, therapy, or device is effective, safe, and useful for human applications. These studies help to find which therapeutic approaches are best for certain diseases. Clinical trial supplies management is necessary for evading overproduction, oversupply, and inventory expiration.
The rising prevalence of chronic diseases boosts the growth of the clinical trial supplies market.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Clinical Trial Supplies Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Clinical Trial Supplies Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Clinical Trial Supplies Market Drivers and Opportunities
High Incidence of Chronic Diseases to Favor Market
The high incidence of chronic diseases in Asia is a key factor likely to contribute to the surge in the number of clinical trials in Asia. According to the World Health Organization (WHO), non-communicable diseases cause 55% of the total deaths, ~8 million every year, in the region. In addition, pharmaceutical companies are likely to conduct drug trials for treating gastroesophageal or liver cancer in China or Korea, respectively, as these countries have many patients suffering from these diseases. Moreover, clinical trials in Asia cost ~30–40% less than in the US and the EU as the doctor visits and medical treatments and procedures are less pricey in Asian countries. Such a factor is anticipated to increase the clinical trials, thereby contributing to the clinical trial supplies market growth.
Outsourcing Clinical Trial Services
Outsourcing clinical trials from contract manufacturers and service providers provides sufficient time for pharmaceutical drug manufacturers to develop other drug formulations, maintain frequent and constant communication with other pharmaceutical companies, and prevent risks and other benefits. Companies such as Fisher Clinical Services, Inc., PAREXEL International, and Piramal Pharma Solutions offer logistics and distribution services to pharmaceutical and biopharmaceutical companies. Thus, the factors mentioned above are responsible for the growth of the clinical trial supplies market size.
Clinical Trial Supplies Market Report Segmentation Analysis
Key segments that contributed to the derivation of the clinical trial supplies market analysis are product & services and stage.
- Based on products & services, the market is segmented into manufacturing, packaging & labelling, and logistics & distribution. The logistics & distribution segment held the largest share of the market in 2023; the segment is anticipated to register the highest CAGR in the market during the forecast period. The logistics and distribution segment is estimated to be the largest segment by product and service, owing to the rising contract manufacturing in the pharmaceutical industry. The cost required for the clinical trial process is much higher. Pharmaceutical and biopharmaceutical companies invest a lot in strict handling of pharmaceutical operations during clinical trials. Thus, various pharmaceutical companies outsource their clinical trials to avoid the unnecessary handling costs due to overproduction, oversupply, and inventory expiration. Thus, the factors are responsible for the growth of the clinical trials supply & logistics market.
- Based on stage, the clinical trial supplies market is segmented into phase I, phase II, phase III, and bioequivalence studies. The Phase III segment held the largest share of the market in 2023 and is estimated to register the highest CAGR in the market during the forecast period. Phase III clinical trial stage is done for large patient groups. The phase III clinical trial stage assists in determining the short-term and long-term efficacy of the active pharmaceutical ingredients. Thus, the assessment is done for the formulated drug's total and associated therapeutic values. As many patients are involved in the clinical trials of phase III, it is important to maintain the efficacy, safety, and accuracy of the drug, which otherwise may result in adverse effects of the drugs in many patients registered for the phase III clinical trials. Shertech Manufacturing is one such company that offers phase III clinical trial services, and it offers comprehensive, definitive data related to efficacy and side effects to its customers. It also assists in complying with FDA standards that further assist in introducing the drug into the market and completing the required licensing applications. Thus, pain management contributes to the clinical trial supplies market and is expected to continue the trend during the forecast period.
Clinical Trial Supplies Market Share Analysis by Geography
The geographic scope of the clinical trial supplies market report is mainly divided into five regions: North America, Asia Pacific, Europe, the Middle East & Africa, and South & Central America.
Based on geography, the clinical trials supplies market is divided into five key regions: North America, Europe, Asia Pacific, South & Central America, and Middle East & Africa. The North American, clinical trials supplies market has been analyzed based on three major countries — the US, Canada, and Mexico. The US clinical trials supply market is estimated to hold the largest market share during the forecast period. The US clinical trial supplies market growth is attributed to the presence of market players in the region that offer manufacturing, storage, logistics, and other services, which is likely to enhance the market growth in the country. For instance, Alderley Analytical, Almac, and others are well-known manufacturing organizations offering a wide range of integrated services to more than 600 pharmaceutical and biotech companies. Additionally, the rise in demand for services and medicines in areas of orphan and rare diseases is expected to encourage drug manufacturers to develop smart medicines and recruit patients for clinical trials. The increasing urge for the development of new drugs in the country is also expected to increase the number of industry entrants by keeping up with the fast pace of the pharmaceutical industry.
Clinical Trial Supplies Market Regional Insights
The regional trends and factors influencing the Clinical Trial Supplies Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Clinical Trial Supplies Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Clinical Trial Supplies Market
Clinical Trial Supplies Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 3819.36 Million |
| Market Size by 2031 | US$ 8085.87 Million |
| Global CAGR (2024 - 2031) | 9.9% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Product & Service
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Clinical Trial Supplies Market Players Density: Understanding Its Impact on Business Dynamics
The Clinical Trial Supplies Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Clinical Trial Supplies Market are:
- Thermo Fisher Scientific
- Catalent
- Eurofins Scientific
- Piramal Pharma Solutions
- PRA Health Sciences
- Marken (a subsidiary of UPS)
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Clinical Trial Supplies Market top key players overview
Clinical Trial Supplies Market News and Recent Developments
The clinical trial supplies market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. The following is a list of developments in the market for innovations, business expansion, and strategies:
- In May 2020, Sharp, part of UDG Healthcare, purchased a pharmaceutical packaging facility from Quality Packaging Specialists International (QPSI) in the US. Situated in Macungie, Pennsylvania, the 160,000ft² facility provides primary and secondary pharmaceutical packaging services to its customers. The Macungie facility provides bottling, blistering, vial labeling, medical device kitting, and sterilization services (Source: Sharp, Press Release)
- In April 2021, Catalent added cryogenic capabilities at its Philadelphia clinical supply services facility. This expansion helped increase Catalent's capabilities in gene therapies, packaging, labeling, and distribution of cryogenic materials for clinical trials (Source: Catalent, Inc., Press Release)
Clinical Trial Supplies Market Report Coverage and Deliverables
The “Clinical Trial Supplies Market Size and Forecast (2021–2031)” report provides a detailed analysis of the market covering the following areas:
- Clinical Trial SuppliesMarket size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Market dynamics such as drivers, restraints, and key opportunities
- Clinical Trial Supplies Market trends
- Detailed PEST/Porter’s Five Forces and SWOT analysis
- Clinical Trial Supplies market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Clinical Trial SuppliesIndustry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments
- Detailed company profiles
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product & Service ; Stage ; Drug Type ; Application , and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
 Get Free Sample For
Get Free Sample For