Microbiology CRO Services Market Growth and Analysis by 2031
Microbiology CRO Services Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Application (Clinical, Medical Device, and Others), Service Type (Assay Development, Custom Viral Stock Production, Microbial Testing, and Others), Microorganisms (Bacteria, Fungi, Viruses, and Parasites), End User (Biotech and Pharmaceutical Companies, Medical Device Companies, and Others), and Geography
Historic Data: 2021-2022 | Base Year: 2023 | Forecast Period: 2024-2031- Report Date : Oct 2024
- Report Code : TIPRE00039340
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 227
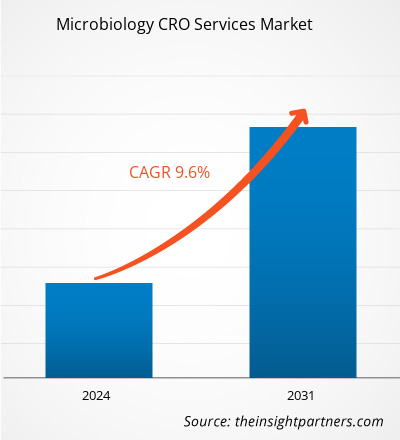
The Microbiology CRO Services market size is projected to reach US$ 11.51 billion by 2031 from US$ 5.52 billion in 2023. The market is expected to register a CAGR of 9.6% during 2023–2031. The integration of artificial intelligence and machine learning is likely to act as a future trend in the market.
Microbiology CRO Services Market Analysis
Microbial testing CROs offer specialized services, including bacterial identification, antimicrobial susceptibility testing, sterility testing, bioburden testing, and molecular testing, to support these research and development efforts. The increasing demand for personalized medicine, targeted therapies, and implantable devices is also increasing the need for microbial testing CROs to provide customized testing services. Thus, increasing research and development investments fuel the microbiology CRO market growth. Moreover, the increasing number of clinical trials and the practice of outsourcing trials to CROs is contributing to the market growth.
Microbiology CRO Services Market Overview
Germany is expected to register the highest CAGR in the microbiology CRO services market in Europe during the forecast period. Germany is expected to be among the significant contributors to the growth of the microbiology CRO services market owing to the increasing number of clinical trials. According to Clinical Trials Arena, Germany held ~3.9% of the total clinical trials carried out worldwide in 2021. Germany has a massive patient pool and a high demand for quality healthcare. Coordinating centers for clinical trials were set up as part of a new funding program under the Federal Ministry of Education and Research to further encourage academic clinical research. The Federal Institute for Drugs and Medical Devices or the Paul-Ehrlich Institute approves clinical trials in Germany, depending on the product to be investigated. Sofpromed, a full-service CRO, states that Germany is considered one of the best countries in the EU to run clinical trials (within the top three countries in Europe in a number of clinical studies). By June 2021, the US clinical trial portal (clinicaltrials.gov) had registered 21,470 clinical studies with at least one German site. Microbiological testing is crucial in Europe for ensuring quality control, regulatory compliance, and minimizing the risk of microbial contamination.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONMicrobiology CRO Services Market: Strategic Insights

-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Microbiology CRO Services Market Drivers and Opportunities
Rising Practice of Outsourcing Clinical Studies with Massive Number of Trials Drive Market Growth
According to the National Library of Medicine (NLM), ~52,000 new studies were registered with NLM (ClinicalTrials.gov) in 2020, and the number increased to ~58,000 in 2023. In January 2023, the NLM reported 38,837 active clinical trials in the US and 105,172 active trials worldwide. According to the European Medicine Agency, in the European Union (EU), ~4,000 clinical trials are authorized annually, of which about 60% of studies are associated with the pharmaceutical industry. An upsurge in the number of clinical trials can be attributed to the rising prevalence of chronic diseases worldwide, which creates an immense demand for more effective treatments.
The increasing complexity of clinical trials makes the proper execution and monitoring of operations carried out in research-based organizations crucial. To avoid errors due to improper execution, many research-based organizations outsource clinical trials to clinical research organizations (CROs). Furthermore, CROs assist in successfully implementing clinical trials by offering deep subject matter expertise at high-quality facilities. They have gradually become an important part of the clinical trial industry through their efficient and cost-effective operations that benefit trial sponsors. According to a blog published by Thermo Fisher Scientific, in 2022, CROs executed ~3 out of 4 clinical trials to reassure the clinical programs of drug developers; provide a wealth of expertise; drive time and cost efficiencies; and deliver customized, high-quality data. Thus, the increasing number of clinical trials and the practice of outsourcing trials to CROs to boost cost-effectiveness and reduce errors drive the microbiology CRO market.
Advancements in Microbiology Testing Technology to Offer Opportunities for Microbiology CRO Services Market
The advancements in microbiology testing technology have revolutionized the pharmaceutical and medical device industries. These advancements enable faster, more accurate, and cost-effective detection of microorganisms. A few critical advancements in microbiology testing technology are mentioned below:
• Molecular Diagnostics: Next-generation sequencing (NGS) and polymerase chain reaction (PCR) technologies have enabled rapid detection of microorganisms, allowing for earlier identification of contamination and reduced product recall rates.
• Automated Systems: Automated systems, such as microbiology analyzers and robotic systems, have increased efficiency, reduced labor costs, and improved accuracy in testing.
• Rapid Microbiological Methods: Techniques such as ATP bioluminescence, PCR-based methods, and mass spectrometry have accelerated microbial identification, reducing the time of results from days to hours or even minutes.
• Microbial Identification Systems: Advanced microbial identification systems such as matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry have improved accuracy and speed in identifying microorganisms.
The abovementioned advancements in microbiology testing technology have improved the accuracy, speed, and efficiency of testing. These improvements enable the pharmaceutical and medical device industries to reduce product recall rates, improve product quality, enhance patient safety, increase efficiency and reduce costs, and accelerate product development and launch timelines. This, in turn, has opened up new avenues for pharmaceutical companies to ensure the quality and safety of their products and for medical device manufacturers to ensure the sterility and biocompatibility of their devices.
Microbiology CRO Services Market Report Segmentation Analysis
Key segments that contributed to the derivation of the microbiology CRO services market analysis are service type, application, microorganisms, and end user.
- Based on service type, the microbiology CRO services market is segmented into assay development, custom viral stock production, microbial testing, and others. The assay development segment held the largest share of the market in 2023.
- By application, the microbiology CRO services market is segmented into clinical, medical device, and others. The clinical segment held the largest share of the market in 2023.
- In terms of microorganisms, the microbiology CRO services market is categorized into bacteria, fungi, viruses, and parasites. The bacteria segment dominated the market in 2023.
- Based on end user, the market is segmented into biotech and pharmaceutical companies, medical device companies, and others. The biotech and pharmaceutical companies segment held the largest share of the market in 2023.
Microbiology CRO Services Market Share Analysis by Geography
The geographic scope of the microbiology CRO services market report is mainly divided into five regions: North America, Asia Pacific, Europe, South & Central America, and the Middle East & Africa. North America dominated the market in 2023. The microbiology CRO market in North America is segmented into the US, Canada, and Mexico. The US is the largest microbiology CRO market and is estimated to dominate the market during the forecast period. The microbiology CRO market growth in the US is driven by the adoption of modern technologies by CROs, the surging number of clinical trials, and increasing investment in research and development by pharmaceutical and biotechnological companies. Also, strategic initiatives by market players play a significant role in supporting the microbiology CRO market growth. For instance, in February 2024, ICON plc acquired Clinical Research Management, Inc. (ClinicalRM). ClinicalRM offers comprehensive research solutions to various US government agencies. The company has extensive expertise in basic and applied research, infectious diseases, vaccine development, testing, and responding to bio-threats. They have collaborated with both government and commercial customers to address the threat of global viral epidemics.
Microbiology CRO Services
Microbiology CRO Services Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 5.52 Billion |
| Market Size by 2031 | US$ 11.51 Billion |
| Global CAGR (2023 - 2031) | 9.6% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Application
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Microbiology CRO Services Market Players Density: Understanding Its Impact on Business Dynamics
The Microbiology CRO Services Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Microbiology CRO Services Market News and Recent Developments
The microbiology CRO services market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. A few of the developments in the microbiology CRO services market are listed below:
- Frontage Laboratories, Inc. and its subsidiary, Frontage Canada, Inc., acquired Nucro-Technics Inc. and its affiliate, Nucro-Technics Holdings, Inc., a pharmaceutical contract research organization offering various services. (Source: Frontage Laboratories, Inc; Company Website; August 2023)
- Pacific BioLabs announced the launch of In Vitro Services. These In Vitro Services offer in vitro, cell-based assays supporting the pharmaceutical, biopharma, and medical device industries. The addition of this group to the PBL testing portfolio increases and is complimentary to well-established testing capabilities in our Microbiology, Analytical/Bioanalytical, and In Vivo departments. (Source: Pacific BioLabs, Company Website, May 2022)
Microbiology CRO Services Market Report Coverage and Deliverables
The "Microbiology CRO Services Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Microbiology CRO services market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Microbiology CRO services market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Microbiology CRO services market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the microbiology CRO services market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





 Get Free Sample For
Get Free Sample For