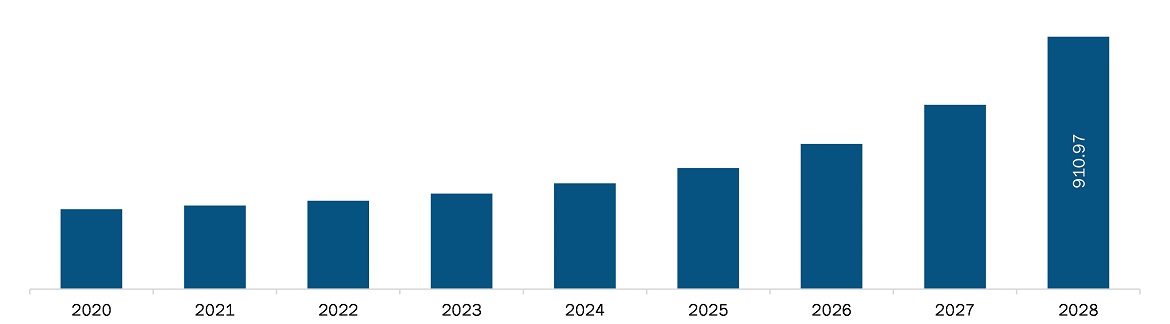
The brain cancer diagnostics market in North America is expected to grow from US$ 301.5 million in 2021 to US$ 911.0 million by 2028; it is estimated to grow at a CAGR of 17.1% from 2021 to 2028.
According to the American Association of Neurological Surgeons, glioblastoma is the most common malignant brain and other CNS tumor, accounting for 47.7% of all cases. The incidence of glioblastoma is 3.21 per 100,000 people. Early detection of tumors often provides more treatment options. Advanced imaging technology can pinpoint the location of brain tumors. Diagnostic tools include magnetic resonance imaging (MRI) and computed tomography (CT or CAT scan). Intraoperative MRI can also be used to guide tissue biopsy and tumor resection during surgery. Magnetic resonance spectroscopy (MRS) is used to examine the chemical characteristics of tumors. Thus, the increase in the prevalence of brain cancer highly demands brain cancer diagnostic tools.
The COVID-19 pandemic has disrupted healthcare systems, leading to concerns about its subsequent impact on non-COVID disease conditions. Cancer diagnosis and treatment are time-sensitive and are likely to be significantly affected by these conditions. The activities of each cancer discipline have been adversely affected by the COVID-19 pandemic. In addition, childhood malignant brain tumors are characterized by rapid growth and require early diagnosis and appropriate treatment. Therefore, delaying or modifying treatment can compromise its effectiveness and reduce patient survival. Flores et al. reported that delayed diagnosis of brain tumors is correlated with a worse prognosis. A prolonged pre-diagnostic symptomatic interval has negative and severe consequences, such as death or serious brain damage following the increase in intracranial pressure. Due to the fear and pressure of SARS CoV2 infection, changes in the decision-making process of children with brain tumors may have a negative impact on their final outcome. Never have multidisciplinary tumor boards serve a crucial role during the current COVID-19 outbreak. All pediatric cases diagnosed with a new brain tumor should be discussed in the multidisciplinary tumor board to determine the best treatment plan for each child.
With the new features and technologies, vendors can attract new customers and expand their footprints in emerging markets. This factor is likely to drive the brain cancer diagnostics market. The North America brain cancer diagnostics market is expected to grow at a good CAGR during the forecast period.
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
North America Brain Cancer Diagnostics Market Segmentation
By Diagnostic Type
- Imaging Test
- MRI
- CT Scan
- PET
- Biopsy
- Molecular Testing
- Lumbar Puncture
- Others
By Cancer Type
- Glioblastoma Multiforme
- Astrocytomas
- Ependymomas
- Others
By End User
- Hospitals
- Specialty Clinics
- Ambulatory Surgical Centers
- Others
By Country
- North America
- US
- Canada
- Mexico
Companies Mentioned
- Thermo Fisher Scientific Inc.
- Siemens Healthineers A
- GE Healthcare
- MDxHealth
- NantOmics
- Biocept, Inc.
- Koninklijke Philips N.V
- Canon Medical Systems
- Hitachi, Ltd.
- Neusoft Medical Systems
North America Brain Cancer Diagnostics Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 301.5 Million |
| Market Size by 2028 | US$ 911.0 Million |
| CAGR (2021 - 2028) | 17.1% |
| Historical Data | 2019-2020 |
| Forecast period | 2022-2028 |
| Segments Covered |
By Diagnostic Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For