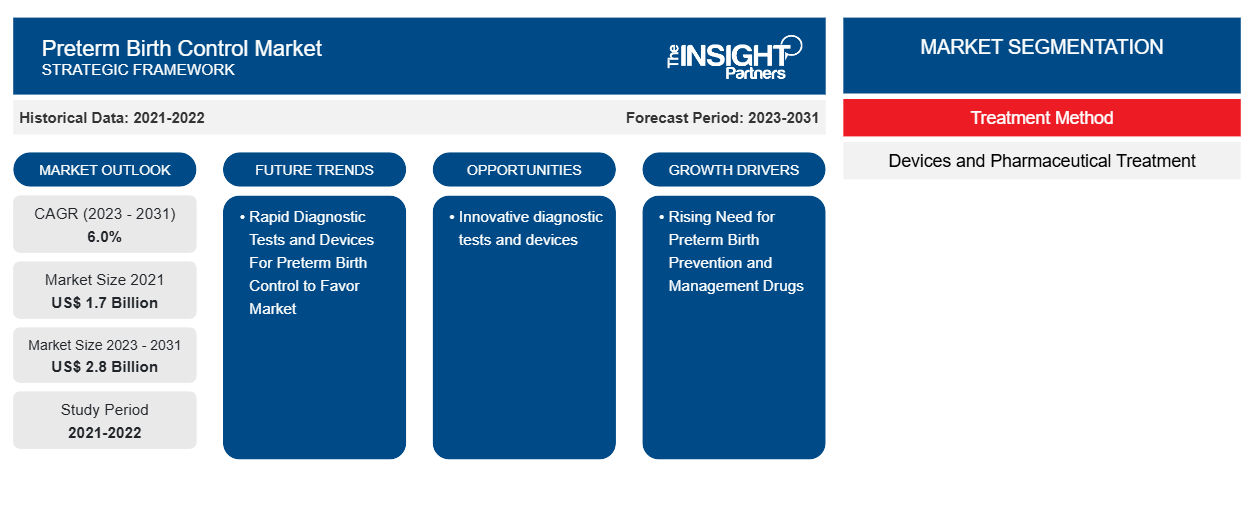
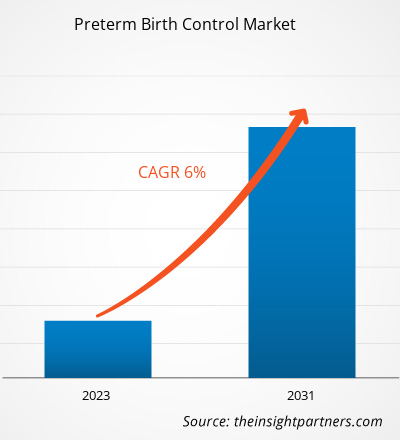
The Preterm Birth ControlMarket was valued at US$ 1.7 billion in 2021 and is expected to reach US$ 2.8 billion by 2031. The market is expected to register a CAGR of 6.0% from 2023–2031. Introduction of novel diagnostic tests and devices is likely to remain key Preterm Birth Control Market trends.
Preterm Birth ControlMarket Analysis
Rising Need for Preterm Birth Prevention and Management Drugs
Preterm birth is when a baby is born too early, before 37 weeks of pregnancy. Also, babies born too early (before 32 weeks) have higher rates of death and disability. According to the Centers for Disease Control and Prevention (CDC) report, in 2022, preterm birth affected almost 1 in every 10 infants born in the US. Therefore, there is a rising need to manage preterm birth. For example, recommendations include progesterone treatment for women having risk of preterm delivery, therapeutic medications, and diagnostic methods. The most common medicines for preterm labor include "Antenatal corticosteroids (also called ACS)." The ACS includes medicines such as betamethasone and dexamethasone. Additionally, preterm labor can be prevented by antibiotics. Antibiotics include "Ceftriaxone," "Clarithromycin," and "Metronidazole." Therefore, through innovative therapeutics, medical devices, and screening methods preterm birth can be effectively managed thereby driving the market growth during 2021-2031.
Preterm Birth ControlMarket Overview
Technology, innovation, and smart technological solutions such as continue to influence Preterm Birth Control Market significantly. Rising need for preterm birth prevention and management drugs and rapid diagnostic tests and devicesare the most influential factors responsible for Preterm Birth Control Market growth. Innovative diagnostic tests and devices is a key trend for Preterm Birth Control Market growth. Preterm birth control product innovationswill provide lucrative market opportunity for the Preterm Birth Control Market growth.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Preterm Birth Control Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Preterm Birth Control Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Preterm Birth ControlMarket Drivers and Opportunities
Rapid Diagnostic Tests and Devices For Preterm Birth Control to Favor Market
Preterm birth is a leading cause of infant mortality, with newborns often facing life-long issues such as breathing problems, cerebral palsy, and intellectual disabilities. Also, preterm labor accounts for huge healthcare costs. For example, in Europe, the healthcare cost associated with preterm birth control accounts for US$21.3 billion, and US$23 billion in the US. Therefore, developing innovative medical devices and diagnostic tests can overcome challenges and healthcare costs associated with preterm labor. For example, "Pregnolia System Device" manufactured by Pregnolia is a novel diagnostic device that can determine the risk of premature birth by detecting cervical tissue stiffness. The device is safe, user-friendly, and more efficient in predicting risk than current ultrasound technology devices. Additionally, PreTRM's "PreTRM Test" is the first predictive test that identifies a pregnant woman's risk of spontaneous preterm birth to improve outcomes even if they lack evident risk factors. The PreTRM Test is the only clinically validated and commercially available blood test that provides early, individualized risk assessment for spontaneous preterm birth in asymptomatic, singleton pregnancies.
Preterm Birth Control Product Innovations – An Opportunity
Healthcare delivery is rapidly developing, and manufacturers have made significant efforts in the past to expedite the speed and accuracy of diagnostic testing across healthcare settings for preterm birth. In July 2021, Organon and ObsEva announced a collaboration to develop and commercialize "Ebopiprant (OBE022)", an Investigational Agent to evaluate first-in-class treatment for preterm labor. The Ebopiprant is an investigational, orally active, selective prostaglandin F2α (PGF2α) receptor agonist evaluated as a potential treatment for preterm labor, reducing the inflammation and uterine contractions. Therefore, innovative product innovation will provide lucrative market opportunities will account considerable market share in the coming years for preterm birth control market.
Preterm Birth Control
Market Report Segmentation Analysis
Key segments that contributed to the derivation of the Preterm Birth Control Market analysis are candidature and services.
- Based on treatment method, the Preterm Birth Control Market is divided into devices and pharmaceutical treatment. The pharmaceutical treatment segment may hold a larger market share in 2023.
Preterm Birth Control Market Share Analysis by Geography
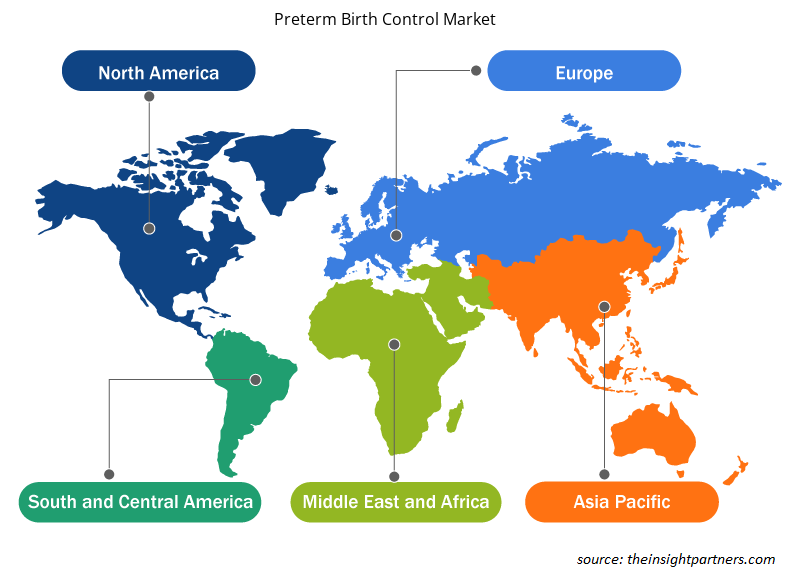
The geographic scope of the Preterm Birth ControlMarket report is mainly divided into five regions: North America, Asia Pacific, Europe, Middle East & Africa, and South America/South & Central America.
North America has dominated the Preterm Birth Control Market. In North America, US accounts considerable share for preterm birth control. Presence of top medical device companies present in the US, therapeutic interventions, and manufacturers inorganic strategic developments are the most influential factors responsible for market growth. Asia Pacific is anticipated to grow with the highest CAGR in the coming years.
Preterm Birth Control
Preterm Birth Control Market Regional Insights
Preterm Birth Control Market Regional Insights
The regional trends and factors influencing the Preterm Birth Control Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Preterm Birth Control Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Preterm Birth Control Market
Preterm Birth Control Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 1.7 Billion |
| Market Size by 2031 | US$ 2.8 Billion |
| Global CAGR (2023 - 2031) | 6.0% |
| Historical Data | 2021-2022 |
| Forecast period | 2023-2031 |
| Segments Covered |
By Treatment Method
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
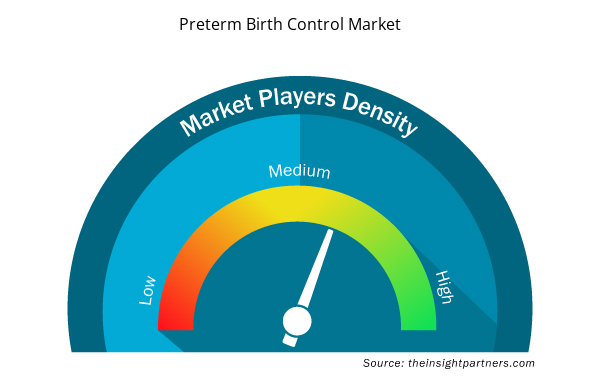
Preterm Birth Control Market Players Density: Understanding Its Impact on Business Dynamics
The Preterm Birth Control Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Preterm Birth Control Market are:
- Cooper Surgical
- Medgyn Products
- Integra Lifesciences Corporation
- Panpac Medical Corp.
- Ar. Arabin GmbH & Co. KG
- Amag Pharmaceuticals
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Preterm Birth Control Market top key players overview
Preterm Birth ControlMarket News and Recent Developments
The Preterm Birth ControlMarket is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. The following is a list of developments in them market for Preterm Birth Control and strategies:
- In December 2023, Sera Prognostics Inc. announced the "Data Safety Monitoring Board (DSMB)" for the pivotal detection of Premature Risk Assessment Combined with Clinical Interventions for Improved Neonatal Outcomes (PRIME) study. The PRIME study will advance and improve to save lives and improve overall health for mothers and newborns worldwide.
Preterm Birth ControlMarket Report Coverage and Deliverables
The “Preterm Birth ControlMarket Size and Forecast (2021–2031)” report provides a detailed analysis of the market covering below areas:
- Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Market dynamics such as drivers, restraints, and key opportunities
- Key future trends
- Detailed PEST/Porter’s Five Forces and SWOT analysis
- Global and regional market analysis covering key market trends, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments
- Detailed company profiles
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Treatment Method , and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
US, Canada, Mexico, UK, Germany, Spain, Italy, France, India, China, Japan, South Korea, Australia, UAE, Saudi Arabia, South Africa, Brazil, Argentina
 Get Free Sample For
Get Free Sample For