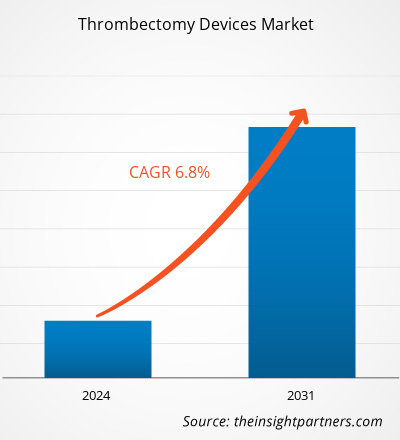
The Thrombectomy Devices Market size was estimated to be US$ 1409.82 million in 2021 and US$ XX million in 2023 and is expected to reach US$ 2708.48 million by 2031; it is estimated to record a CAGR of 6.8% in till 2031. The market's growth is attributed to key driving factors such as the elevating incidences of CVDs and neurological diseases, technological advancements, government initiatives towards prevention of diseases, and favorable medical reimbursement scenario Such factors are likely to remain key Thrombectomy Devices Market trends.
Thrombectomy Devices Market Analysis
Neurological diseases are disorders of brain, spine, and the nerves connecting the earlier two as well as supplying oxygenated blood. The neurovascular systems are highly dependent on the continuous supply of oxygen and nutrients supplied by the arteries and veins. Therefore, a defect in the system can impair the function, and it may quickly become a life-threatening issue. Thrombosis has been increasing in diseases such as peripheral vascular, neurovascular, and other diseases. Thrombosis is a biological response that is closely linked to cerebral aneurysms, which are balloon-like dilations of blood vessel walls, caused by the weakening of the wall layers.
The prevalence of cerebral aneurysms has increased excessively across the globe. ~30,000 people in the US suffer from brain aneurysm rupture every year. The brain aneurysm breaks every 18 minutes, and the annual rate of rupture in the US is found close to 8–10 per 100,000 people. Moreover, peripheral vascular diseases (PVD) decrease the blood flow and affect blood vessels outside of the heart and brain, and the most commonly affected parts of the body are legs and feet. This disease—a coronary artery disease—occurs in people facing problems with the arteries, which supply blood to heart. Thus, the increasing incidences of target patients and the severity of these conditions are likely to fuel the growth of the thrombectomy devices market during the forecast period.
Thrombectomy Devices Market Overview
Surgical thrombectomy is a surgery performed for the removal of a blood clot from an artery or vein. The surgery is performed when the blood coagulates and clusters to form a blood clot in the blood vessels. In operation, an incision is made using thrombectomy devices in a blood vessel. The clot is eliminated, and the blood vessel is fixed; it repairs blood flow. Mechanical thrombectomy devices are most widely used for thrombectomy procedures as compared to the other methods.
Global thrombectomy devices market is segmented by region into North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America. In North America, the US is the largest market for thrombectomy devices. The US is estimated to hold the largest thrombectomy devices market share during the forecast period. The growth of the thrombectomy devices market in this country is attributed to a rising number of apheresis procedures at a growing numbers of apheresis centers, and access to the well-developed infrastructure. The growth of the market is ascribed to the growing advancements in the thrombectomy devices. The country has well-developed healthcare facility centers equipped with modern-age equipment and instruments. It is also experiencing increasing incidences of chronic diseases such as neurological conditions, cardiovascular diseases, and renal diseases. The abovementioned factors are expected to fuel the growth of the market in the US. The US is among the highly advanced countries having various technologies available for a robust medical infrastructure, and it is globally known for its involvement in the research and development pertaining to the therapeutic techniques. The country serves as a home for several national and international companies, which contributes significantly to the medical device market. Several companies in the country offer thrombectomy devices.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Thrombectomy Devices Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Thrombectomy Devices Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Thrombectomy Devices Market Drivers and Opportunities
Rising Rate of Peripheral Arterial Diseases (PAD) to Favor the Overall Market
According to the Centers for Diseases Control and Prevention (CDC), the incidence rate of peripheral arterial diseases (PADs) is narrowing the vessels that carry blood from the heart to the legs. Men and women are equally affected by PAD. However, the black ethnicity has been reported to be at a greater risk of getting PADs. ~8.5 million people in the US have PAD, including 12–20% of individuals that are older than 60 years of age. However, only 25% of the population is estimated to be aware of PADs. Limited awareness increases complications such as costs and delays the commencement of rehabilitation. This can be avoided, and the impact can be minimized with an early detection and appropriate management. Both neurointerventionists and stroke specialists need to be aware of the risk factors, strategies for prevention, and management of such complications.
Such a trend is likely to drive the overall thrombectomy devices market during the forecast period.
Technological Advancements in Devices and Procedures is Expected to Propel the Market Demand – An Opportunity of Thrombectomy Devices Market
Accelerated advances in devices and procedures have propelled the development of thrombectomy procedure over the decade, from rudimentary mechanical disruption, followed by intra-arterial thrombolytic infusions, to increasingly effective thrombectomy devices. Further, thrombectomy is being increasingly used in new therapeutic areas to treat acute stroke, DVT, pulmonary embolism (PE), and venous thromboembolism (VTE). Regulatory and reimbursement systems play a key role in creating an idea that promotes the adoption of thrombectomy devices for stroke or other diseases. Nonetheless, at present, the number of regulatory and reimbursement schemes for the neurovascular therapies is increasing significantly is increasing significantly, thereby boosting the neurovascular thrombectomy devices market.
Such advancements in recent years is likely to generate attractive growth opportunity for the thrombectomy devices market.
Thrombectomy Devices Market Report Segmentation Analysis
Key segments that contributed to the derivation of the Thrombectomy Devices Market analysis are product and application.
- Based on product, the thrombectomy devices market is segmented into mechanical thrombectomy devices, rheolytic thrombectomy devices, aspiration thrombectomy devices, and ultrasonic thrombectomy devices. Mechanical thrombectomy devices segment held the largest share of the market, and the segment is expected to register the highest CAGR in the market during the forecast period. Recombinant cell lines are preferred systems for the production of therapeutic recombinant proteins. Mechanical thrombectomy is an interventional procedure used for the removal of blood clots (thrombus) from a main cerebral artery or blood vessel. The method is also known as embolectomy and is used in restoring blood flow after acute ischemic stroke. The mechanical thrombectomy process involves a variety of endovascular tools for removing, fragmenting, or dispersing thrombus in veins, arteries, or bypass grafts. Based on their mode of action, and related advantages and also disadvantages, the Mechanical Devices are offered in two types, namely, stent retrievers and aspiration catheters. Various applications of mechanical thrombectomy include neurovascular, peripheral vascular, and cardiovascular, among other procedures. Thus, the growing adoption of mechanical thrombectomy devices is likely to grow the demand of the segment during the forecast period.
- Based on application, the thrombectomy devices market is segmented into neurovascular, peripheral vascular, cardiovascular and others. In 2023, the cardiovascular segment held the largest share of the market. However, the peripheral vascular segment is also expected to register the highest CAGR in the market during the forecast period. Heart attacks generally develop as a result of coronary artery disease. Thrombectomy is a catheter-based procedure that involves targeting of these blood clots. There are several cardiovascular devices available in the market include a stent, cardiac catheter, guidewire, heart valve, and many more. Peripheral vascular disease (PVD) is a blood circulation disorder that causes the brain to block, narrow, or spasm. The procedure can be used to remove thrombi and emboli from vessels of the venous systems and peripheral arterial. The minimally-invasive devices are used to enable the rapid restoration of blood flow in such cases as deep vein thrombosis and acute limb ischemia. Moreover, acute embolism and thrombosis are the most common cause of peripheral vascular occlusion, thereby boosting the peripheral thrombectomy.
Thrombectomy Devices Market Share Analysis by Geography
The geographic scope of the Thrombectomy Devices Market report is mainly segmented into five regions: North America, Asia Pacific, Europe, the Middle East & Africa, and South America/South & Central America.
Asia Pacific accounted as the fastest growing region in the global thrombectomy devices market and was projected to grow at a faster pace over the forecast period. The growth of the thrombectomy devices market share in this region is primarily due to rising geriatric population, increasing chronic diseases, and other factors. In addition, growing healthcare expenditure is likely to increase the growth opportunities during the coming years. The market in the region is largely held by countries such as China and Japan. The countries such as Australia, India, and South Korea are estimated to serve various growth opportunities due to the rising development in the healthcare sector, in line with the rising healthcare expenditure. In addition, the governments of India and Australia are increasing their efforts to supply thrombectomy products across the country. Also, the rise in the incidence of CVDs is likely to provide greater growth opportunities to the market players in the coming years.
Thrombectomy Devices Market Regional Insights
The regional trends and factors influencing the Thrombectomy Devices Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Thrombectomy Devices Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Thrombectomy Devices Market
Thrombectomy Devices Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2021 | US$ 1409.82 Million |
| Market Size by 2031 | US$ 2708.48 Million |
| Global CAGR (2023 - 2031) | 6.8% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Thrombectomy Devices Market Players Density: Understanding Its Impact on Business Dynamics
The Thrombectomy Devices Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Thrombectomy Devices Market are:
- Penumbra, Inc.
- Stryker Corporation
- Medtronic, Inc.
- Edward Lifesciences Corporation
- Terumo Corporation
- Koninklijke Philips N.V
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Thrombectomy Devices Market top key players overview
Thrombectomy Devices Market News and Recent Developments
The Thrombectomy Devices Market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. The following is a list of developments in the market for thrombectomy devices and strategies:
- In January 2023, Penumbra, Inc., a global healthcare company, announced the approval and launch of Lightning FlashTM. It is the most advanced and powerful mechanical thrombectomy system on the market. Lightning Flash incorporates Penumbra's breakthrough Lightning Intelligent Aspiration technology, which currently includes two clot detection algorithms. In conjunction with innovative catheter technology, Lightning Flash is intended to quickly remove large blood clots in the body, such as venous thrombus and pulmonary embolism (PE) (Source: Penumbra, Inc., Press Release, 2023)
Thrombectomy Devices Market Report Coverage and Deliverables
The “Thrombectomy Devices Market Size and Forecast (2021–2031)” report provides a detailed analysis of the market covering below areas:
- Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Market dynamics such as drivers, restraints, and key opportunities
- Key future trends
- Detailed PEST/Porter’s Five Forces and SWOT analysis
- Global and regional market analysis covering key market trends, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments
- Detailed company profiles
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends
Yes! We provide a free sample of the report, which includes Report Scope (Table of Contents), report structure, and selected insights to help you assess the value of the full report. Please click on the "Download Sample" button or contact us to receive your copy.
Absolutely — analyst assistance is part of the package. You can connect with our analyst post-purchase to clarify report insights, methodology or discuss how the findings apply to your business needs.
Once your order is successfully placed, you will receive a confirmation email along with your invoice.
• For published reports: You’ll receive access to the report within 4–6 working hours via a secured email sent to your email.
• For upcoming reports: Your order will be recorded as a pre-booking. Our team will share the estimated release date and keep you informed of any updates. As soon as the report is published, it will be delivered to your registered email.
We offer customization options to align the report with your specific objectives. Whether you need deeper insights into a particular region, industry segment, competitor analysis, or data cut, our research team can tailor the report accordingly. Please share your requirements with us, and we’ll be happy to provide a customized proposal or scope.
The report is available in either PDF format or as an Excel dataset, depending on the license you choose.
The PDF version provides the full analysis and visuals in a ready-to-read format. The Excel dataset includes all underlying data tables for easy manipulation and further analysis.
Please review the license options at checkout or contact us to confirm which formats are included with your purchase.
Our payment process is fully secure and PCI-DSS compliant.
We use trusted and encrypted payment gateways to ensure that all transactions are protected with industry-standard SSL encryption. Your payment details are never stored on our servers and are handled securely by certified third-party processors.
You can make your purchase with confidence, knowing your personal and financial information is safe with us.
Yes, we do offer special pricing for bulk purchases.
If you're interested in purchasing multiple reports, we’re happy to provide a customized bundle offer or volume-based discount tailored to your needs. Please contact our sales team with the list of reports you’re considering, and we’ll share a personalized quote.
Yes, absolutely.
Our team is available to help you make an informed decision. Whether you have questions about the report’s scope, methodology, customization options, or which license suits you best, we’re here to assist. Please reach out to us at sales@theinsightpartners.com, and one of our representatives will get in touch promptly.
Yes, a billing invoice will be automatically generated and sent to your registered email upon successful completion of your purchase.
If you need the invoice in a specific format or require additional details (such as company name, GST, or VAT information), feel free to contact us, and we’ll be happy to assist.
Yes, certainly.
If you encounter any difficulties accessing or receiving your report, our support team is ready to assist you. Simply reach out to us via email or live chat with your order information, and we’ll ensure the issue is resolved quickly so you can access your report without interruption.





















 Get Free Sample For
Get Free Sample For