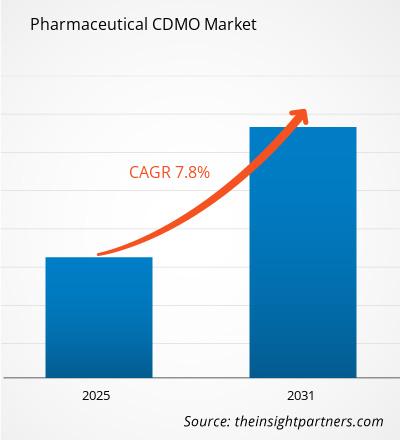
The Pharmaceutical CDMO Market is expected to register a CAGR of 7.8% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Product (API, Drug Product), By Workflow (Clinical, Commercial), By Application (Oncology, Infectious Diseases, Neurological Disorders)
Purpose of the Report
The report Pharmaceutical CDMO Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Pharmaceutical CDMO Market Segmentation
Product
- API
- Drug Product
Workflow
- Clinical
- Commercial
Application
- Oncology
- Infectious Diseases
- Neurological Disorders
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONPharmaceutical CDMO Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Pharmaceutical CDMO Market Growth Drivers
- Increasing Demand for Outsourced Drug Manufacturing: The rising complexity and cost of drug development have led pharmaceutical companies to increasingly rely on Contract Development and Manufacturing Organizations (CDMOs) for specialized services. CDMOs offer cost-effective, efficient, and scalable solutions, allowing pharmaceutical companies to focus on core competencies. This trend is fueling growth in the pharmaceutical CDMO market, particularly for the manufacturing of both small molecules and biologics.
- Growth in Biopharmaceuticals and Complex Drugs: The increasing focus on biologics, biosimilars, and personalized medicines is driving demand for specialized manufacturing capabilities. CDMOs with expertise in biopharmaceuticals are in high demand as pharmaceutical companies require advanced manufacturing platforms to produce biologics, gene therapies, and monoclonal antibodies. As the demand for complex drugs rises, CDMOs play a critical role in meeting these needs, fueling market expansion.
- Regulatory Compliance and Global Expansion: Pharmaceutical companies are seeking CDMOs with global regulatory expertise and the ability to meet international compliance standards. The increasing complexity of regulations, particularly for biologics and new drug formulations, drives the demand for CDMOs that can navigate these requirements. Additionally, the global expansion of the pharmaceutical industry increases the need for CDMOs that can deliver high-quality products across diverse markets, contributing to market growth.
Pharmaceutical CDMO Market Future Trends
- Shift Toward Integrated Services: The trend toward integrated services in the pharmaceutical CDMO market is gaining traction, where companies provide end-to-end solutions from drug development to manufacturing and commercialization. Integrated services streamline the process, reduce timelines, and lower costs for pharmaceutical companies. CDMOs offering a comprehensive range of services, including formulation development, clinical trials, and commercialization, are becoming more attractive to clients seeking efficiency and cost savings.
- Emphasis on Digitalization and Automation: The adoption of digital technologies and automation in pharmaceutical manufacturing is transforming the CDMO landscape. Automation enhances the efficiency of drug production, improves quality control, and reduces operational costs. The increasing use of data analytics, artificial intelligence, and machine learning to optimize manufacturing processes is driving innovation and improving productivity, making CDMOs more competitive and responsive to customer needs.
- Outsourcing of Analytical and Packaging Services: Pharmaceutical companies are increasingly outsourcing analytical testing and packaging services to CDMOs. This trend allows pharmaceutical firms to focus on their core competencies, such as research and development, while ensuring that testing and packaging requirements are met by experts. CDMOs with specialized capabilities in analytical testing, packaging, and labeling are becoming more valuable partners for pharmaceutical companies looking to streamline their operations.
Pharmaceutical CDMO Market Opportunities
- Expansion into Emerging Markets: Emerging markets, such as China, India, and Latin America, present significant growth opportunities for the pharmaceutical CDMO market. As these regions experience rapid industrialization and healthcare improvements, the demand for affordable, high-quality pharmaceutical manufacturing services is increasing. CDMOs can capitalize on this growth by establishing a presence in these markets to offer their services to local pharmaceutical companies and international firms expanding into these regions.
- Growth in Biosimilars and Generic Drugs: The increasing adoption of biosimilars and generic drugs presents a key opportunity for pharmaceutical CDMOs. As patent expirations for major drugs increase, there is a growing demand for cost-effective generic and biosimilar alternatives. CDMOs with the expertise to manufacture these products, including complex biologics and small-molecule generics, are well-positioned to capture market share in this rapidly expanding segment.
- Advancements in Cell and Gene Therapies: The development of cell and gene therapies is opening new opportunities for pharmaceutical CDMOs. As the pharmaceutical industry shifts toward cutting-edge treatments for rare diseases and genetic disorders, CDMOs with the capabilities to handle complex manufacturing processes for gene therapies, cell therapies, and advanced biologics are in high demand. This emerging field offers significant growth potential for CDMOs equipped with the right technology and expertise.
Pharmaceutical CDMO Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 7.8% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Product
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Pharmaceutical CDMO Market Players Density: Understanding Its Impact on Business Dynamics
The Pharmaceutical CDMO Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Pharmaceutical CDMO Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Pharmaceutical CDMO Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
Frequently Asked Questions
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends







 Get Free Sample For
Get Free Sample For