Sterile Compounding Pharmacies Market Share, Size and Opportunities by 2034
Sterile Compounding Pharmacies Market Size and Forecast (2021 - 2034), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product (Injectable Drugs and Infusions) and Route of Administration (Intravenous, Intramuscular, and Subcutaneous), and Geography
Historic Data: 2021-2024 | Base Year: 2025 | Forecast Period: 2026-2034- Report Date : Mar 2026
- Report Code : TIPRE00026302
- Category : Life Sciences
- Status : Upcoming
- Available Report Formats :


- No. of Pages : 150
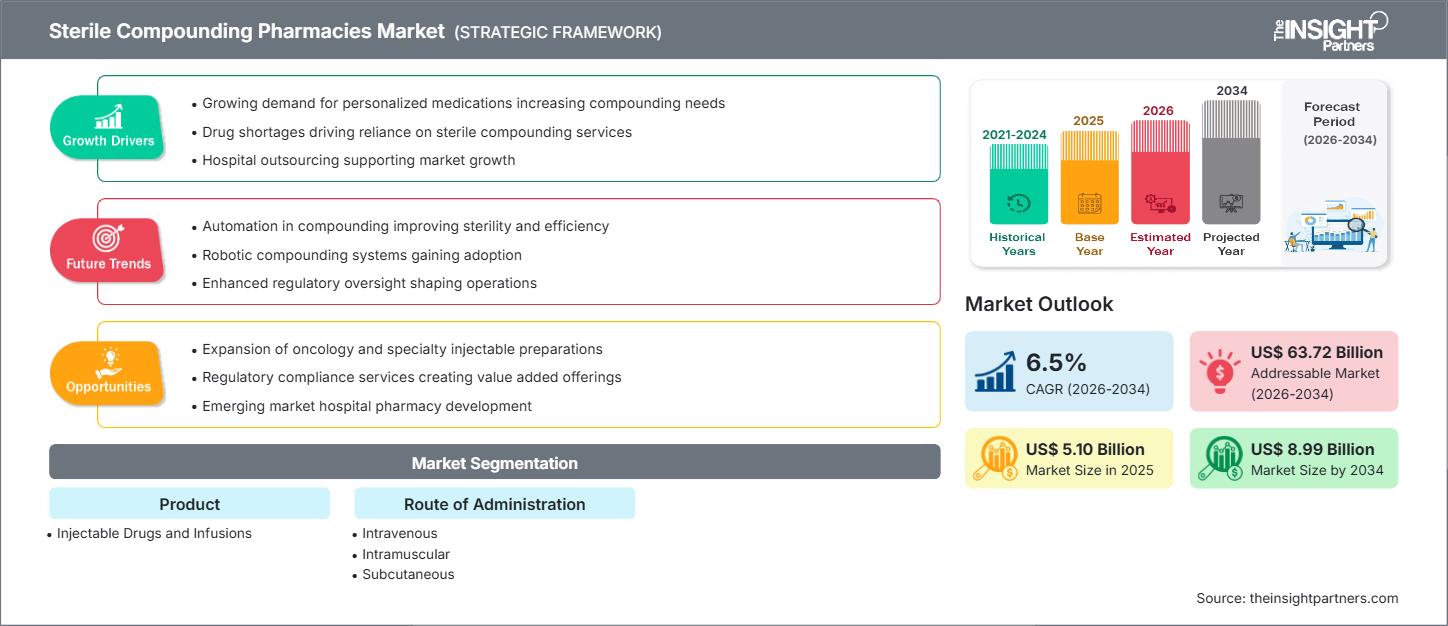
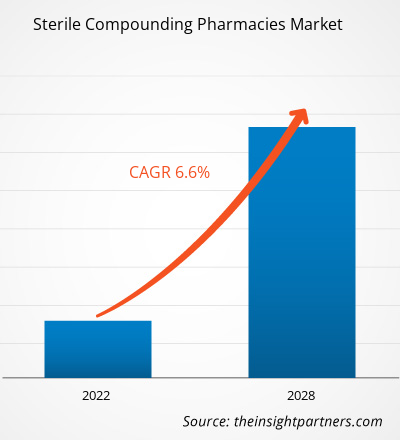
The global sterile compounding pharmacies market size is expected to reach US$ 8.99 billion by 2034 from US$ 5.10 billion in 2025. The market is anticipated to register a CAGR of 6.5% during 2026–2034.
Sterile Compounding Pharmacies Market Analysis
The sterile compounding pharmacies market is undergoing a significant transformation driven by the escalating demand for high-precision, personalized medicine and the outsourcing of complex sterile preparations by hospital networks. Market analysis shows that strategic growth is concentrated in oncology and clinical nutrition, where standardized dosages often do not address the needs of pediatric or geriatric patients. The market is moving toward '503B' outsourcing facilities in the U.S. and similar regulated organizations in other countries. Hospitals are choosing these options to lower the risks and costs of running their own cleanrooms that must meet changing USP <797> and <800> standards. Strategic opportunities are emerging in the integration of automated robotic compounding systems, which not only enhance aseptic integrity but also provide the data-driven throughput necessary to handle the rising volume of specialized injectable drug shortages.
Sterile Compounding Pharmacies Market Overview
The sterile compounding pharmacies market is a critical part of healthcare, preparing customized medications free from microorganisms and pyrogens. The industry follows strict aseptic processing standards for products like intravenous admixtures, intramuscular injections, and subcutaneous therapies. More companies are moving from manual preparation to advanced technology-based services to improve patient safety for those receiving chemotherapy, total parenteral nutrition, and specialized pain management. As chronic diseases needing injectable therapies become more common, sterile compounding pharmacies help acute care settings by enabling hospitals to provide tailored pharmaceutical care while maintaining financial stability and regulatory compliance.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONSterile Compounding Pharmacies Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Sterile Compounding Pharmacies Market Drivers and Opportunities
Market Drivers:
- Rising Demand for Operational Efficiency in Healthcare Delivery: Healthcare organizations are observing more patients who need complex injectable therapies, making in-house pharmacy work more complicated. As a result, more providers are outsourcing high-risk preparation tasks to sterile compounding services to keep operations running smoothly.
- Growing Demand for Personalized Medications and Injectable Therapies: As precision medicine becomes more common, the need for sterile compounding has grown. Tailored dosages for oncology and critical care are now a regular part of healthcare, not just a specialized service.
- Increasing Government Initiatives and Regulatory Mandates: Sterile compounding solutions are subject to widespread implementation of safety standards like the Drug Quality and Security Act (DQSA). Healthcare providers are being incentivized to adopt professional compounding services to move away from high-risk, non-compliant in-house processes.
Market Opportunities:
- Expansion in Emerging Markets with Growing Private Healthcare Sectors: Emerging markets such as India, China, and Brazil are seeing greater adoption of sterile compounding as private hospital networks expand and investments in specialty care clinics rise.
- Integration with Automation and Robotic Compounding Platforms: Integrating sterile compounding with advanced robotics enables seamless, error-free drug preparation. Using AI-driven automation improves clinical decisions and system efficiency through intelligent volume analytics.
- Demand for Specialty Care Segments and Home Infusion Therapy: The trend toward home-based healthcare infrastructure is a main driver of the demand for outsourced sterile infusions. These services enable workflow optimization and set the efficient utilization of resources for long-term patient care.
Sterile Compounding Pharmacies Market Report Segmentation Analysis
The sterile compounding pharmacies market share is analyzed across various segments to provide a clearer understanding of its structure, growth potential, and emerging trends. Below is the standard segmentation approach used in most industry reports:
By Product:
- Injectable Drugs
- Infusions
By Route of Administration:
- Intravenous (IV)
- Intramuscular
- Subcutaneous
By Geography:
- North America
- Europe
- Asia Pacific
- South & Central America
- Middle East & Africa
Sterile Compounding Pharmacies Market Regional Insights
The regional trends and factors influencing the Sterile Compounding Pharmacies Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Sterile Compounding Pharmacies Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Sterile Compounding Pharmacies Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2025 | US$ 5.10 Billion |
| Market Size by 2034 | US$ 8.99 Billion |
| Global CAGR (2026 - 2034) | 6.5% |
| Historical Data | 2021-2024 |
| Forecast period | 2026-2034 |
| Segments Covered |
By Product
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Sterile Compounding Pharmacies Market Players Density: Understanding Its Impact on Business Dynamics
The Sterile Compounding Pharmacies Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Sterile Compounding Pharmacies Market top key players overview
Sterile Compounding Pharmacies Market Share Analysis by Geography
Asia Pacific is expected to grow fastest in the coming years. Emerging markets in South & Central America, the Middle East, and Africa also have many untapped opportunities for outsourcing facilities and specialized compounding providers to expand.
The sterile compounding pharmacies market shows a different growth trajectory in each region due to factors such as regulatory rigor (e.g., USP <797> compliance), hospital outsourcing trends, and the rising demand for personalized parenteral nutrition and oncology treatments. Below is a summary of market share and trends by region:
North America
- Market Share: Holds the largest market share due to advanced healthcare infrastructure and early adoption of 503B outsourcing models.
- Key Drivers:
- Many organizations follow USP <797> and <800> standards to meet regulatory requirements.
- There is a strong demand for specialized IVs that are ready to use for oncology and pain management.
- Large outsourcing facilities, such as QuVa Pharma and Nephron, play a major role in the market.
- Trends: A strong shift toward robotic compounding and automated workflow coordination to eliminate human error and reduce contamination risks in high-volume facilities.
2. Europe
- Market Share: Driven by public healthcare systems and strict safety governance, with a high emphasis on hospital-based compounding.
- Key Drivers:
- National digitization projects aimed at unifying electronic health records with pharmacy orders
- Growing need for efficiency in multi-specialty hospital practices to manage drug shortages
- Increasing adoption of "centralized compounding hubs" to serve multiple clinics
- Trends: Rising use of interoperable platforms to support cross-border health initiatives and the standardization of aseptic preparation protocols across the EU.
3. Asia Pacific
- Market Share: Fastest-growing region owing to rapid healthcare modernization and expanding medical tourism in China, India, and Japan.
- Key Drivers:
- Government programs like "Digital India" and "Healthy China 2030" are driving change in the sector.
- More private healthcare providers are now investing in their own sterile suites.
- Chronic diseases that need long-term parenteral therapy are becoming more common.
- Trends: Rapid adoption of automated compounding systems for appointment-driven specialty medication delivery, particularly in large urban pediatric and oncology centers.
4. South and Central America
- Market Share: Emerging market with a focused transition toward local production of personalized sterile medications.
- Key Drivers:
- Modernization of private hospital networks in Brazil, Mexico, and Colombia
- Regulatory evolution (e.g., INVIMA and ANVISA) is pushing for higher aseptic standards.
- High demand for customized hormone replacement and anti-aging therapies
- Trends: Increasing reliance on global technology transfers (e.g., from providers like Fagron) to establish compliant sterile labs that meet high safety standards for local hospitals.
5. Middle East and Africa
- Market Share: Developing market with significant growth potential, particularly within the GCC's advanced medical cities.
- Key Drivers:
- National e-health strategies and the rise of "Smart Hospitals" in Saudi Arabia and the UAE
- Investment in localized biopharma and compounding infrastructure to secure the supply chain
- Rising demand for specialized pediatric and neonatal sterile formulations
- Trends: Implementation of specialized robotic suites in newly built "Health Cities" to support precision medicine and reduce reliance on imported specialty injectables.
Sterile Compounding Pharmacies Market Players Density: Understanding Its Impact on Business Dynamics
High Market Density and Competition
Competition is intensifying due to the presence of global pharmaceutical and compounding leaders such as Fagron, Inc., B. Braun Melsungen AG, and Fresenius Kabi AG. The landscape is further shaped by prominent 503B outsourcing facilities and specialized regional providers like Avella Specialty Pharmacy and Pace Pharmacy (Canada), creating a densely populated market focused on high-stakes clinical compliance.
This competitive environment pushes vendors to differentiate through:
- Advanced Robotic Automation: Leveraging IV compounding robotics and automated dispensing systems to eliminate human error, enhance throughput, and ensure the highest precision in sterile preparations.
- Rigorous Regulatory Alignment: Investing heavily in USP <797> and USP <800> compliant infrastructure, including modular cleanrooms and continuous environmental monitoring to guarantee data integrity and patient safety.
- Personalized Biologics and Injectables: Developing patient-specific formulations for oncology, parenteral nutrition, and rare diseases that commercial manufacturers cannot provide in standard dosages.
- Digital Traceability Solutions: Implementing blockchain-enabled tracking and electronic batch records to provide real-time visibility across the production lifecycle, from ingredient sourcing to final delivery.
Opportunities and Strategic Moves
- Partner with large hospital networks and surgery centers to shift from in-house mixing to specialized 503B outsourcing, reducing overhead and helping address local drug shortages.
- Use AI-driven quality control and predictive inventory tools to improve the supply of important sterile admixtures, especially for high-demand areas such as anesthesia and intensive care.
Major Companies operating in the Sterile Compounding Pharmacies Market are:
- Fagron, Inc. – Netherlands
- B. Braun Melsungen AG – Germany
- Fresenius Kabi AG – Germany
- PharMedium Healthcare Holdings, Inc. – United States
- Avella Specialty Pharmacy – United States
- Triangle Compounding Pharmacy – United States
- Pencol Compounding Pharmacy – United States
- Pavilion Compounding Pharmacy, LLC – United States
- Pace Pharmacy – Canada
Disclaimer: The companies listed above are not ranked in any particular order.
Sterile Compounding Pharmacies Market News and Recent Developments
- In September 2025, Medisca partnered with Stevanato Group to distribute EZ-fill® ready-to-use sterile vials, expanding access to high-quality containment solutions for the pharmaceutical compounding industry, improving operational efficiency, and patient safety.
- In December 2025, Wolters Kluwer introduced its Advanced Compounding module for Simplifi 797, offering sterile compounding pharmacies a comprehensive solution to meet updated USP Chapter 797 standards. The module supports high-risk sterile processes, batch tracking, beyond-use dating, and enhanced documentation, while providing integrated training and compliance resources for pharmacy staff.
Sterile Compounding Pharmacies Market Report Coverage and Deliverables
The "Sterile Compounding Pharmacies Market Size and Forecast (2021–2034)" report provides a detailed analysis of the market covering below areas:
- Sterile Compounding Pharmacies Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Sterile Compounding Pharmacies Market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Sterile Compounding Pharmacies Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments in the Sterile Compounding Pharmacies Market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For