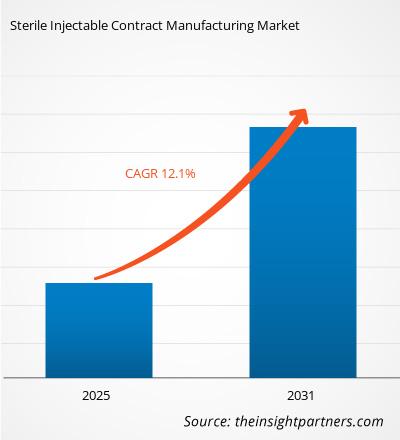
The Sterile Injectable Contract Manufacturing Market is expected to register a CAGR of 12.1% from 2025 to 2031, with a market size expanding from US$ XX million in 2024 to US$ XX Million by 2031.
The report is segmented by Molecule Type (Small Molecule, Large Molecule). The report further presents analysis based on Therapeutic Application (Cancer, Diabetes, Cardiovascular Diseases, Central Nervous System Diseases, Infectious Disorders, Musculoskeletal, Anti-viral, Others). The report is further segmented based on Route of Administration (Subcutaneous, Intravenous, Intramuscular, Others). Futher, it is segmented based on End-use (Pharmaceutical Companies, Biopharmaceutical Companies, Others). The global analysis is further broken-down at regional level and major countries. The report offers the value in USD for the above analysis and segments.
Purpose of the Report
The report Sterile Injectable Contract Manufacturing Market by The Insight Partners aims to describe the present landscape and future growth, top driving factors, challenges, and opportunities. This will provide insights to various business stakeholders, such as:
- Technology Providers/Manufacturers: To understand the evolving market dynamics and know the potential growth opportunities, enabling them to make informed strategic decisions.
- Investors: To conduct a comprehensive trend analysis regarding the market growth rate, market financial projections, and opportunities that exist across the value chain.
- Regulatory bodies: To regulate policies and police activities in the market with the aim of minimizing abuse, preserving investor trust and confidence, and upholding the integrity and stability of the market.
Sterile Injectable Contract Manufacturing Market Segmentation
Molecule Type
- Small Molecule
- Large Molecule
Therapeutic Application
- Cancer
- Diabetes
- Cardiovascular Diseases
- Central Nervous System Diseases
- Infectious Disorders
- Musculoskeletal
- Anti-viral
Route of Administration
- Subcutaneous
- Intravenous
- Intramuscular
End-use
- Pharmaceutical Companies
- Biopharmaceutical Companies
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONSterile Injectable Contract Manufacturing Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Sterile Injectable Contract Manufacturing Market Growth Drivers
- Rising Demand for Biologics and Biosimilars: The increasing adoption of biologics and biosimilars in treating various chronic conditions is propelling the demand for sterile injectable contract manufacturing. Biologic drugs often require complex manufacturing processes and specialized facilities, leading pharmaceutical companies to partner with experienced contract manufacturers for scalability and quality assurance.
- Cost Efficiency: Outsourcing sterile injectable manufacturing offers cost efficiencies for pharmaceutical companies. By leveraging the established infrastructure, technological advancements, and workforce of contract manufacturers, companies can avoid high capital investments in specialized production facilities and focus on their core competencies, improving overall profitability.
- Aging Population and Chronic Diseases: The global increase in the elderly population and the prevalence of chronic diseases such as diabetes, cancer, and cardiovascular disorders are creating a greater need for injectable medications. Contract manufacturers help meet this growing demand by scaling up production of injectables, ensuring timely supply to the global market.
Sterile Injectable Contract Manufacturing Market Future Trends
- Adoption of Advanced Technologies: The future of sterile injectable contract manufacturing lies in the integration of advanced technologies such as automated production lines, AI-driven quality control, and continuous manufacturing processes. These innovations will improve production efficiency, reduce errors, and shorten time-to-market, making sterile injectables more accessible.
- Focus on Single-Use Systems: The adoption of single-use systems (SUS) is becoming a prominent trend in sterile injectable manufacturing. SUS minimizes the risk of cross-contamination, reduces downtime between batches, and lowers cleaning and sterilization costs, making it an attractive option for contract manufacturers to meet the rising demand for injectables.
- Personalized Medicine and Cell Therapies: As personalized medicine and cell therapies gain traction, the demand for small-batch, high-quality sterile injectables is increasing. This trend is pushing contract manufacturers to invest in specialized capabilities to produce niche products, such as personalized biologics and gene therapies, which require precision manufacturing processes.
Sterile Injectable Contract Manufacturing Market Opportunities
- Partnerships and Strategic Collaborations: Ongoing opportunities exist in forming strategic partnerships between pharmaceutical companies and contract manufacturers to co-develop and manufacture new injectable drugs. Collaborative efforts can help streamline drug development, reduce production costs, and increase the speed to market for critical therapies.
- Growing Demand for Pre-filled Syringes: The market for pre-filled syringes, which are gaining popularity due to their ease of use and convenience, presents a significant opportunity for contract manufacturers. As patient self-administration of injectables increases, the demand for pre-filled syringes will drive the need for specialized manufacturing services to meet this trend.
- Focus on Cold Chain Logistics: As the number of temperature-sensitive injectable products, including biologics and vaccines, continues to rise, there is an opportunity for contract manufacturers to expand cold chain capabilities. Investment in reliable, efficient cold chain solutions ensures the safe and timely delivery of sterile injectables to patients worldwide.
Sterile Injectable Contract Manufacturing Market Regional Insights
The regional trends and factors influencing the Sterile Injectable Contract Manufacturing Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Sterile Injectable Contract Manufacturing Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Sterile Injectable Contract Manufacturing Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ XX million |
| Market Size by 2031 | US$ XX Million |
| Global CAGR (2025 - 2031) | 12.1% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Molecule Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Sterile Injectable Contract Manufacturing Market Players Density: Understanding Its Impact on Business Dynamics
The Sterile Injectable Contract Manufacturing Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Sterile Injectable Contract Manufacturing Market top key players overview
Key Selling Points
- Comprehensive Coverage: The report comprehensively covers the analysis of products, services, types, and end users of the Sterile Injectable Contract Manufacturing Market, providing a holistic landscape.
- Expert Analysis: The report is compiled based on the in-depth understanding of industry experts and analysts.
- Up-to-date Information: The report assures business relevance due to its coverage of recent information and data trends.
- Customization Options: This report can be customized to cater to specific client requirements and suit the business strategies aptly.
The research report on the Sterile Injectable Contract Manufacturing Market can, therefore, help spearhead the trail of decoding and understanding the industry scenario and growth prospects. Although there can be a few valid concerns, the overall benefits of this report tend to outweigh the disadvantages.
Frequently Asked Questions
1. Rising Demand for Biologics and Biosimilars.
2. Aging Population and Chronic Diseases.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For