The Asia Pacific Healthcare contract research organizations (CRO) services market is expected to reach US$ 16,745.94 million by 2027 from US$ 7,559.03 million in 2019; it is estimated to grow at a CAGR of 10.6% from 2020 to 2027.
The growth of the Asia Pacific healthcare contract research organizations (CRO) services market is mainly attributed as growing number of clinical trials and increasing expenditure in R&D and outsourcing activities in the country. However, the dearth of skilled professional is the major factor hindering the market growth in Asia Pacific.
Contract research organization (CRO) provides support services to the pharmaceutical, biotechnology, and medical device companies throughout the development of the product. Pharmaceutical& biotechnology companies and the medical device industry require various testing services such as preclinical services, clinical research services, analytical testing services, bio analytical testing services, and regulatory services. CROs are specifically designed to perform these testing services to reduce the in-house cost and time of the companies, required to conduct the extensive range of testing services to comply with the national and international regulatory standards.
Clinical trials play a determinant role in drug approvals and its efficacy over diseases. The studies help in understanding the best medical approaches in certain therapeutic areas. The healthcare systems are observing a rapid increase in the number of clinical trials. Factors such as growing demand for novel drugs and medical equipment, increasing prevalence of chronic conditions, and growth in funding programs to conduct clinical trials are responsible for propelling the number of clinical trials.
With the consistent and growing demand for clinical trials worldwide, geographic expansion into Asia continues at a steady pace. The need for Asia as a region for clinical trial conduct and clinical trial sites is a result of both the competitive landscape for patients as well as a diversification among sponsor companies to file new drug applications in the Asian markets, requiring local trials and patients. China represents one of the largest potential growth regions for clinical trials and CRO market in the next five years if the CFDA reforms are enacted and start-up timelines decrease significantly. The access to large numbers of patients, both disease-specific and treatment naïve, in urban settings will continue to make China attractive for trials. The remaining Southeast Asian countries (Malaysia, Philippines, Cambodia, Vietnam, Myanmar, Thailand, Laos, Indonesia) all represent a small number of active clinical trial sites. However, with respect to populations and access to treatment naïve patients, as well as certain therapeutic indications (tropical disease, infectious disease, certain cancers), these countries present excellent growth opportunities for CROs. Although the cost of conducting clinical trials in Asia is significantly lower. The outsourcing penetration in Japan is just 20% compared to ~50% in the US, so there is significant room for growth.
Asia Pacific countries are expecting to witness massive challenges due to increasing COVID-19. Prohibitory measures have been taken to control the spread of this pandemic. However, in this measure, hospitals, clinics, diagnostic centers, ambulatory surgery centers (ASCs) not taking any general patient on priority. Apart from the overall health system, healthcare contract research organizations (CRO) services have been facing challenges due to coronavirus outbreak. Thus, such cancellations of studies is likely to negatively affect the growth of the market.
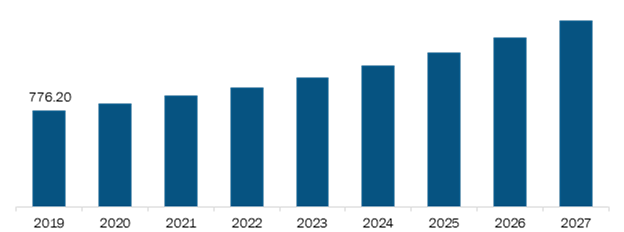
Rest of Asia Pacific Healthcare Contract Research Organizations (CRO) Services Market, Revenue and Forecast to 2027 (US$ Mn)
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
ASIA PACIFIC HEALTHCARE CONTRACT RESEARCH ORGANIZATIONS (CRO) SERVICES MARKET SEGMENTATION
By Service Type
- Early Phase Development
- Laboratory Services
- Consulting Services
- Clinical Research Services
By Therapeutic Application
- Oncology
- Infectious Diseases
- Respiratory Diseases
- Cardiovascular Diseases
- Neurological Disorders
- Others
By End-User
- Medical Device Companies
- Pharmaceutical and Biopharmaceutical Companies
- Others (Academic Institutes)
By Country
- Japan
- China
- South Korea
- India
- Australia
Company Profiles
- Wuxi AppTec
- IQVIA INC
- Laboratory Corporation of America Holdings (Covance)
- PRA Health Sciences
- Syneos Health
Asia Pacific Healthcare Contract Research Organizations (CRO) Services Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2019 | US$ 7,559.03 Million |
| Market Size by 2027 | US$ 16,745.94 Million |
| CAGR (2020 - 2027) | 10.6% |
| Historical Data | 2017-2018 |
| Forecast period | 2020-2027 |
| Segments Covered |
By Service Type
|
| Regions and Countries Covered |
Asia-Pacific
|
| Market leaders and key company profiles |
|
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends






















 Get Free Sample For
Get Free Sample For