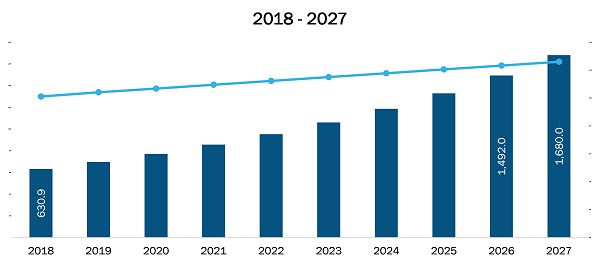
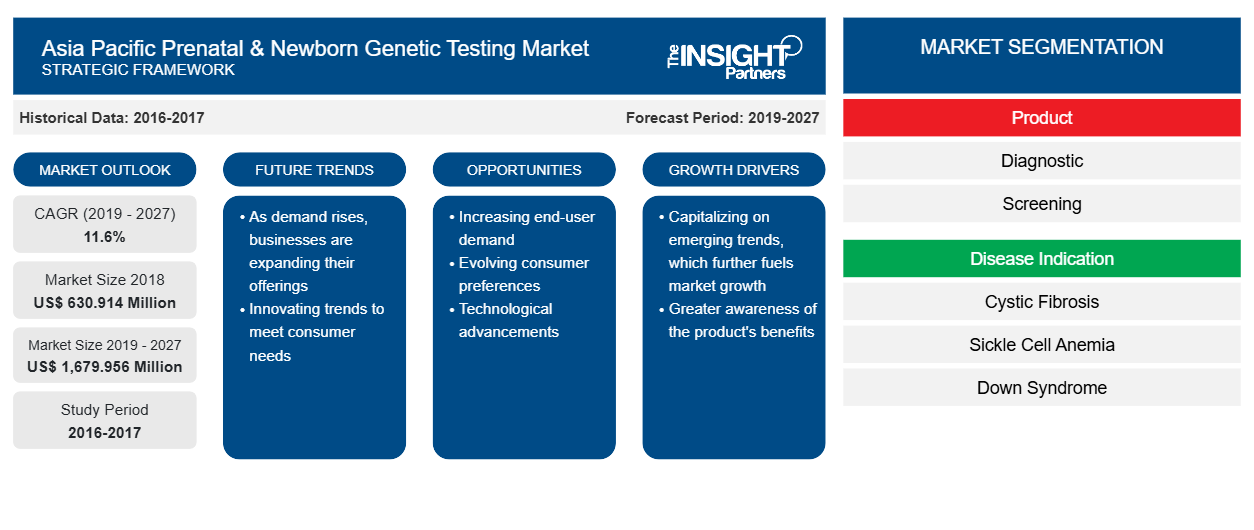
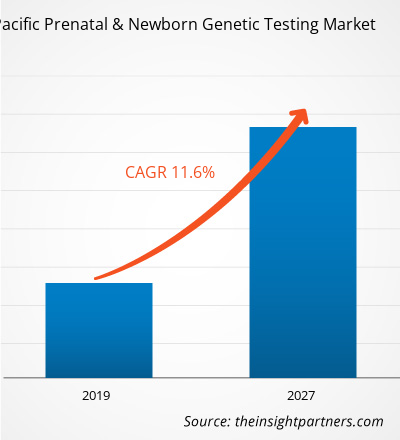
The Asia Pacific prenatal and newborn genetic testing market is expected to reach US$ 1,679.956 Mn in 2027 from US$ 630.914 in 2018. The market is estimated to grow with a CAGR of 11.6% from 2019-2027.
The key factors responsible for the growth of the Prenatal and Newborn Genetic testing market in Asia Pacific are rising burden of genetic diseases among infants, increasing fertility rates and developing healthcare scenario with rising awareness among populace regarding the benefits of prenatal testing. On the other hand, use of digital microfluidics in newborn testing is likely to be a prevalent trend in the future years.
Lucrative Regional Markets
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Market Insights
Burden of Genetic Diseases in India
Several types of genetic diseases affect the fetuses in the womb. The way in which these genetic diseases are inherited helps to determine the risk that they pose on pregnancy as well as the risk of its recurrence. The risk of having genetic diseases in babies is high in cases where the parents have another child with a genetic disease, family history of a genetic disorder, or if either of a parent has a chromosomal abnormality. There is a significant prevalence of genetic diseases among infants. Moreover, these diseases are also responsible for infant mortality across the globe. Five most common genetic diseases in India affecting the new born are Beta-Thalassemia, Cystic Fibrosis, Sickle Cell Anemia, Spinal Muscular Atrophy and Hemophilia A. Sickle cell anemia is inherited blood disorder and is most common among African, Arabian and Indian population. The Indian pediatrics estimated that every year approximately 9,000-10,000 new born suffer from β-thalassemia. Furthermore, in 2011, India reported 14,718 cases with bleeding diseases and 11,586 patients with hemophilia A.
Furthermore, according to the previous study, “The burden of genetic disorders in India and a framework for community control” in 2002, estimated that approximately, 495,000 new-borns with congenital malformations, 21,400 with Down syndrome, 9,000 with beta-thalassemia, 5,200 with sickle cell disease are born each year. Due to presence of large population, high birth rate, consanguineous marriage in various Indian communities, there is a high incidence of genetic diseases in India. Thus, the high prevalence of genetic diseases among infants account for the increasing demands for prenatal and newborn genetic tests, thereby contributing to the growth of the market.
Development of Policy for Treatment of Rare Diseases
Genetic diseases are, in most cases, chronic, devastating and life threatening, which often requires extensive and specialized treatments. Moreover, these diseases results in some form of disability, which may get extremely severe sometimes. These genetic diseases place a huge psychological, physical, and socioeconomic burden on patients as well as their families. The most common rare diseases are hemophilia, thalassemia, sickle-cell anemia, auto-immune diseases, lysosomal storage disorders and others. Approximately 80% of rare diseases have identified to be of genetic origin and hence have an impact on children.
The government of India (GOI) had constituted committees with the aim of framing a ‘national policy on treatment of 17 rare diseases’. Likewise, the government of National Capital Territory (NCT) of Delhi also appointed an interdisciplinary committee for rare diseases. Following are the committees appointed for rare diseases:
- Committee under Professor V.K. Paul, Head, Department of Paediatrics, AIIMS, New Delhi, for ‘Prioritisation of Therapy for Rare Genetic Disorders’
- Sub-committee on rare diseases in India, under Prof. I.C. Verma, Director, Institute of Medical Genetics Genomics, Sir Ganga Ram Hospital – ‘Guidelines for Therapy and Management’
Thus, growing efforts by the Government is likely to boost the market and is expected to provide significant growth opportunities for the prenatal and new born genetic testing market during the forecast period.
Product Insights
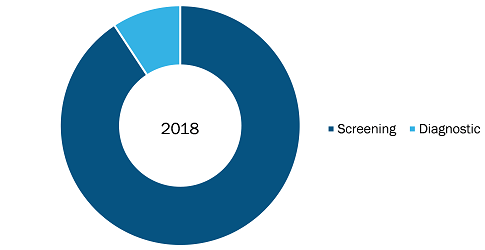
The Asia Pacific prenatal and newborn genetic testing market, based on the product was segmented into screening and diagnostics. In 2018, the screening segment held a largest market share of the prenatal and newborn genetic testing market, by product. Moreover, the same segment is also expected to witness the highest CAGR over the coming years owing to the factors such as increasing number of pregnant females adopting prenatal screening, increased mandatory regulations for newborn screening tests and others. Moreover, screens are the first line tests that pregnant women and newborn undergoes that also adds up to the major share of the market and its dominance in the Asia Pacific market.
Prenatal and newborn genetic testing Market, by Product
- This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Asia Pacific Prenatal & Newborn Genetic Testing Market: Strategic Insights
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Asia Pacific Prenatal & Newborn Genetic Testing Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Partnerships, Product up-gradation and product launches were observed as the most adopted strategy in Asia Pacific prenatal and newborn genetic testing industry. Few of the recent partnerships, product launch and up-gradations are listed below;
2019:
Illumina introduced VeriSeq NIPT Solution v2, a CE-IVD, next-generation sequencing (NGS)-based approach to noninvasive prenatal testing (NIPT). The automated comprehensive solution allows laboratories to screen for a broader range of chromosomal and sub-chromosomal conditions associated with birth defects and adverse pregnancy outcomes than the standard NIPT menu.2017:
Natera, Inc. upgraded its Panorama non-invasive prenatal test (NIPT) to screen twin pregnancies for zygosity (identical or non-identical/fraternal) and chromosomal abnormalities.2015:
SRL Diagnostics has introduced a breakthrough medical test to diagnose hypertensive disorders in pregnancy recently at its state of the art lab in Gurgaon. PLGF (Placental Growth Factor) is used for accurate diagnosis and prognosis of “pre-eclampsia”, a major cause of maternal, foetal, neonatal morbidity and mortality.2017:
LifeCodexx AG, a non-invasive prenatal DNA testing company in Europe and LifeCell announced a partnership to bring PrenaTesT qNIPTTM testing for the first time to India. The qNIPT technology detects the presence of fetal trisomy 21 (Down syndrome) from maternal blood.PRENATAL AND NEWBORN GENETIC TESTING – MARKET SEGMENTATION
By Test Type
- Prenatal Genetic Testing
- Newborn Genetic Testing
By Product
- Screening
- Maternal Serum Screening
- Non-invasive Prenatal Testing
- Diagnostic
- Chronic Villus Sampling
- Amniocentesis
- Cytogenetic and Pharmacogenetic Testing
By Disease Indication
- Cystic Fibrosis
- Sickle Cell Anemia
- Down Syndrome
- Phenyketonuria
- Recurrent Pregnancy Loss
- Antiphospholipid Syndrome
- Other Diseases
By Technology
- Spectrophotometry
- Polymerase Chain Reaction
- Fluorescence in-situ Hybridization
- Array Competitive Genomic Hybridization
- Others
By End User
- Hospitals & Clinics
- Diagnostic Centres
- Other End Users
By Geography
Asia Pacific
- China
- Japan
- India
- Indonesia
- Singapore
- Malaysia
- Thailand
- Pakistan
- Bangladesh
- Sri Lanka
- Nepal
Company Profiles
- LifeCell
- Quest Diagnostics Incorporated
- Medgenome
- Eurofins Scientific
- Metropolis India
- SRL Diagnostics
- Sandor Lifesciences Pvt Ltd
- PerkinElmer, Inc.
- Natera, Inc.
- Laboratory Corporation of America Holdings (Sequenom)
- Genes2Me LLP
- Illumina, Inc.
Asia Pacific Prenatal & Newborn Genetic Testing Market Regional Insights
The regional trends and factors influencing the Asia Pacific Prenatal & Newborn Genetic Testing Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Asia Pacific Prenatal & Newborn Genetic Testing Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
- Get the Regional Specific Data for Asia Pacific Prenatal & Newborn Genetic Testing Market
Asia Pacific Prenatal & Newborn Genetic Testing Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2018 | US$ 630.914 Million |
| Market Size by 2027 | US$ 1,679.956 Million |
| Global CAGR (2019 - 2027) | 11.6% |
| Historical Data | 2016-2017 |
| Forecast period | 2019-2027 |
| Segments Covered |
By Product
|
| Regions and Countries Covered | Asia-Pacific
|
| Market leaders and key company profiles |
Asia Pacific Prenatal & Newborn Genetic Testing Market Players Density: Understanding Its Impact on Business Dynamics
The Asia Pacific Prenatal & Newborn Genetic Testing Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Asia Pacific Prenatal & Newborn Genetic Testing Market are:
- LifeCell
- Quest Diagnostics Incorporated
- Medgenome
- Eurofins Scientific
- Metropolis India
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Asia Pacific Prenatal & Newborn Genetic Testing Market top key players overview
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset



Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Product, Technology, End User and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Australia, China, Japan, South Korea
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Asia Pacific Prenatal and Newborn Genetic Testing Market
- LifeCell
- Quest Diagnostics Incorporated
- Medgenome
- Eurofins Scientific
- Metropolis India
- SRL Diagnostics
- Sandor Lifesciences Pvt Ltd
- PerkinElmer, Inc.
- Natera, Inc.
- Laboratory Corporation of America Holdings (Sequenom)
- Genes2Me LLP
- Illumina, Inc.
 Get Free Sample For
Get Free Sample For