Balloon Aortic Valvuloplasty Market Growth and Recent Trends by 2031
Balloon Aortic Valvuloplasty Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Product Type (Non-Compliant Balloons and Semi-Compliant Balloons) and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)
Historic Data: 2021-2022 | Base Year: 2023 | Forecast Period: 2024-2031- Report Date : Jun 2024
- Report Code : TIPRE00039182
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 160
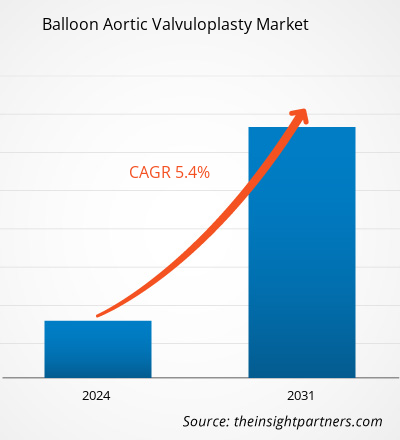
The balloon aortic valvuloplasty market size is projected to reach US$ 190.27 million by 2031 from US$ 125.08 million in 2023. The market is expected to register a CAGR of 5.4% during 2023–2031. Emerging research and development strategies that help improve transcatheter aortic valve implantation (TAVI) are likely to remain key trends in the market.
Balloon Aortic Valvuloplasty Market Analysis
The factors driving the growth of the balloon valvuloplasty device market include the rapidly aging population and the increasing prevalence rate of aortic valve stenosis. Aortic stenosis has become the most common valvular disease that requires surgical treatment. Balloon aortic valvuloplasty (BAV) can be helpful in a wide range of patients with aortic stenosis, as well as being a bridging therapy to valve replacement, besides its role in TAVI procedures. Rising availability of medical reimbursements for balloon valvuloplasty devices, growing adoption of minimally invasive surgeries, and better healthcare infrastructure are additional factors contributing to the market growth. Minimally invasive approaches usually involve smaller incisions with the use of specialized instruments and technologies to access the heart, which can lead to shorter hospital stays and less scarring. Additionally, healthcare organizations are investing in training their medical teams and obtaining the necessary equipment to meet the growing demand for minimally invasive BAV and TAVI surgeries, thereby providing patients with enhanced treatment options and better overall healthcare experiences. Thus, the balloon aortic valvuloplasty market witnesses notable growth.
Balloon Aortic Valvuloplasty Market Overview
More than half of patients suffering from severe heart valve disease die within two years of acquiring the symptoms. Survey results published by Global Heart Hub in Heart Valve Voice Canada 2020 state that only 3% of Canadians aged 60 and above are aware of aortic stenosis. Increased awareness and early detection of heart valve disease are therefore essential for patients, along with those dependent on them and the local community. The Canadian Cardiovascular Society formed a Heart Valve Disease Strategy Working Group to address gaps in heart valve disease care in Canada. In January 2021, Medtronic received an expanded indication from Health Canada for the Evolut TAVI system. Medtronic's TAVI platform is the only system licensed for both bicuspid aortic valves (intermediate or higher risk patients). Evolut is indicated in the country for severe aortic stenosis patients across all risk categories. In September 2021, Abbott received the US Food and Drug Administration (FDA) approval for Portico with FlexNav transcatheter aortic valve replacement (TAVR) system to treat people with symptomatic, severe aortic stenosis who are at high risk for open-heart surgery.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONBalloon Aortic Valvuloplasty Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Balloon Aortic Valvuloplasty Market Drivers and Opportunities
Growing Demand for Minimally Invasive Procedures Favors Market Growth
The soaring demand for minimally invasive techniques in valve replacement surgeries reflects a significant shift in medical practices and patient preferences. In a minimally invasive aortic valvuloplasty, the heart's aortic valve is repaired and, if needed, replaced through TAVI procedures. Minimally invasive techniques are driven by the desire to reduce the invasiveness of procedures, minimize the associated risks, and promote faster recovery times.
All branches of medicine, including cardiology, are witnessing an increase in the demand for less invasive procedures because of several benefits over traditional open-heart surgery. Compared to open-heart surgery, patients who undergo minimally invasive procedures usually have a shorter recovery time. In addition, minimally invasive techniques have a lower risk of complications and result in fewer scars compared to open cardiac surgery. Surgeons have adopted these techniques due to advancements in medical devices, and improved surgical skills can further facilitate widespread adoption. As BAV is a minimally invasive technique that can be used to treat a variety of heart problems, including aortic valve stenosis, the market for BAV devices is driven by the increased demand for minimally invasive procedures.
Advancements in Aortic Valve Replacement Technologies
Patients with heart conditions experience improved symptoms within a shorter period when treated with transcatheter procedures compared with surgical interventions. Minimally invasive surgical techniques, such as TAVI, have modernized the treatment of aortic stenosis due to advancements. Additionally, improvements in design have led to the development of stronger and biocompatible aortic valve replacements, which have enhanced patient outcomes and lowered the risk of complications. A few technological advancements by the companies in balloon aortic valvuloplasty and aortic valve replacement devices are mentioned below:
- In January 2023, Abbott received FDA approval for the Navitor TAVI system to treat patients with severe aortic stenosis and those who are ineligible for open-heart surgery. Navitor has been newly introduced in the company's extensive transcatheter structural heart portfolio; it offers physicians and patients less invasive treatment alternatives for a range of serious heart conditions.
- In September 2020, Boston Scientific introduced the ACURATE neo2 aortic valve system in Europe. The next-generation TAVI technology—a new platform designed with multiple features to improve the clinical performance of the original ACURATE neo platform—had an extended indication for patients with aortic stenosis compared to the aortic valve system of the previous generation.
Therefore, technological advancements are offering lucrative opportunities for the balloon aortic valvuloplasty market growth.
Balloon Aortic Valvuloplasty Market Report Segmentation Analysis
The key segment that contributed to the derivation of the balloon aortic valvuloplasty market analysis is product type.
Based on product type, the balloon aortic valvuloplasty market is bifurcated into non-compliant balloons and semi-compliant balloons. The non-compliant balloons segment held a larger market share in 2023 and is expected to register a higher CAGR in the market during 2023–2031.
Balloon Aortic Valvuloplasty Market Share Analysis by Geography
The geographic scope of the balloon aortic valvuloplasty market report is mainly divided into five regions: North America, Asia Pacific, Europe, Middle East & Africa, and South & Central America.
North America held a significant share of the market. The growth of the market in this region is attributed to the rising prevalence of heart valve diseases, increasing initiatives by various organizations to raise awareness about heart valve diseases among the population, and advancements made in treatment devices. According to the Organization for Economic Co-operation and Development, the burden of cardiovascular diseases is increasing rapidly in Mexico. The risk factors associated with heart diseases among the adult and geriatric population are being addressed in Mexico via awareness programs. In response to these awareness programs, transcatheter aortic valve replacement surgeries have gained traction and are commonly preferred as an alternative to open-heart valve surgery. Cardiologists in Mexico City perform TAVI using the best technology and techniques. CMQ Hospitals is a leader in the TAVI procedure in Puerto Vallarta and Riviera Nayarit. The hospitals have a multidisciplinary team of professionals in interventional cardiology, guaranteeing comprehensive and personalized care for each patient.
The most common cause of aortic stenosis in Canada is degenerative valve disease due to aging, followed by congenital bicuspid aortic valve disease and rheumatic heart disease. As per the “Heart Valve Disease in Canada,” published by the Institute of Health Economics in March 2022, aortic stenosis and mitral regurgitation are the two most common heart valve diseases. About 2.5% of the country’s population is reported to have heart valve disease, which increases significantly after the age of 65, reaching 13% in people aged 75 and above. It is estimated that 1.5 million people aged above 65 will suffer from heart valve disease by 2040 in Canada.
In Canada, a significant number of market players offer TAVI products. Edwards Lifesciences, Boston Scientific Corporation, Medtronic Inc., JenaValve Technology, and Transcatheter Technologies GmbH are a few manufacturers operating in the market. These manufacturers have received regulatory approvals from the Canadian government. Edwards SAPIEN and Medtronic CoreValve systems have been granted licenses in Canada.
Balloon Aortic Valvuloplasty Market Regional Insights
The regional trends and factors influencing the Balloon Aortic Valvuloplasty Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Balloon Aortic Valvuloplasty Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Balloon Aortic Valvuloplasty Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2023 | US$ 125.08 Million |
| Market Size by 2031 | US$ 190.27 Million |
| Global CAGR (2023 - 2031) | 5.4% |
| Historical Data | 2021-2022 |
| Forecast period | 2024-2031 |
| Segments Covered |
By Product Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Balloon Aortic Valvuloplasty Market Players Density: Understanding Its Impact on Business Dynamics
The Balloon Aortic Valvuloplasty Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Balloon Aortic Valvuloplasty Market top key players overview
Balloon Aortic Valvuloplasty Market News and Recent Developments
The balloon aortic valvuloplasty market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. A few of the developments in the balloon aortic valvuloplasty market are listed below:
- Abbott launched its latest-generation TAVI system, Navitor, in India, making the minimally invasive device available for people with severe aortic stenosis who are at high or extreme surgical risk in the country. Navitor features a unique fabric cuff (NaviSeal) that works with the cardiac cycle to minimize or eliminate a backflow of blood across the valve frame, known as paravalvular leak. (Source: Abbott, Press Release, December 2022)
- Keystone Heart, Ltd., entered into an exclusive distribution agreement with InterValve Medical Inc. to sell and market their portfolio of balloon aortic valvuloplasty products in the US. Under the agreement, Keystone Heart Ltd. began selling the V8 and TAV8 aortic valvuloplasty balloon catheters (InterValve Medical) in the US market. (Source: Keystone Heart, Ltd; Press Release, March 2021)
Balloon Aortic Valvuloplasty Market Report Coverage and Deliverables
The “Balloon Aortic Valvuloplasty Market Size and Forecast (2021–2031)” report provides a detailed analysis of the market covering below areas:
- Balloon aortic valvuloplasty market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Balloon aortic valvuloplasty market trends as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Balloon aortic valvuloplasty market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the balloon aortic valvuloplasty market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For