Bulk Lyophilization Services Market Size, Share, Trends, and Forecast to 2031
Bulk Lyophilization Services Market Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Lyophilization Format (Bulk Tray Lyophilization, Drum Lyophilization, Shelf Freeze-Drying, Tunnel or Conveyer Based Freeze Drying, and Hybrid Methods), Scale (Small Scale or Lab Scale, Pilot Scale, and Commercial and Industrial Scale), Service Type (Custom Process Development and Optimization, Pilot Scale and R and D Lyophilization, and Full Scale Commercial Bulk Lyophilization), and End User (Pharmaceutical and Biotechnology Companies, Research and Academic Institutes, and Others), and Geography (North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America)
Historic Data: 2021-2023 | Base Year: 2024 | Forecast Period: 2025-2031- Report Date : Dec 2025
- Report Code : TIPRE00041488
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 238
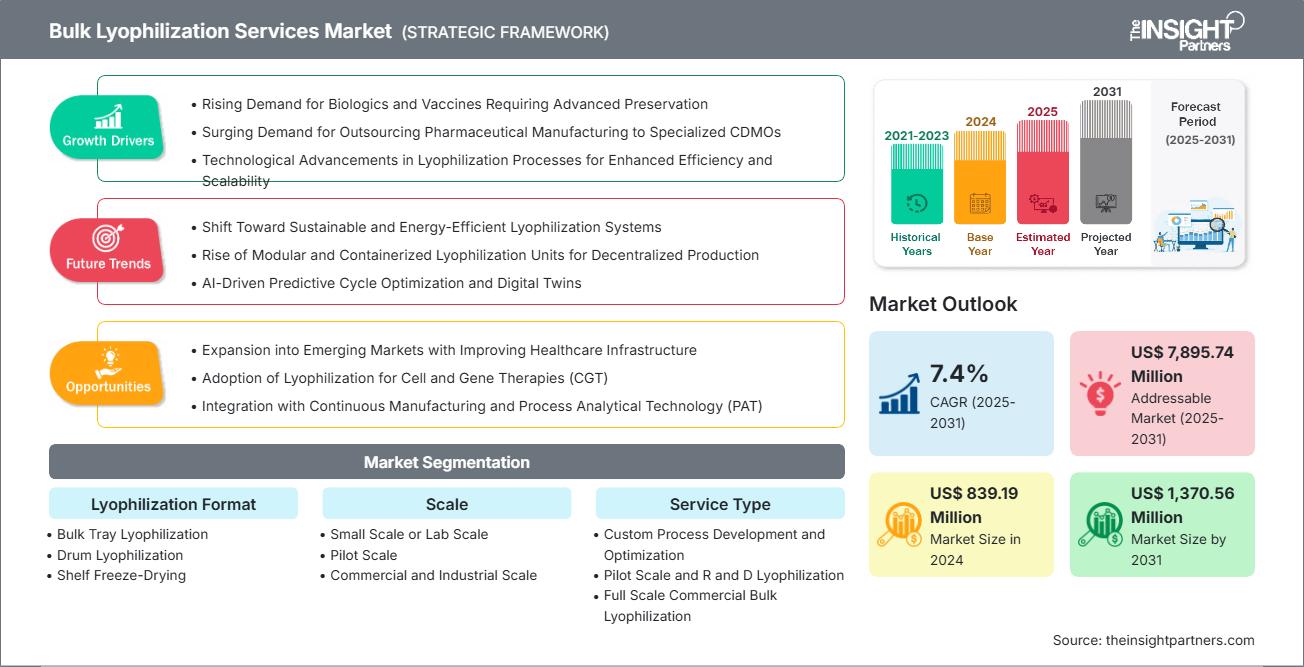
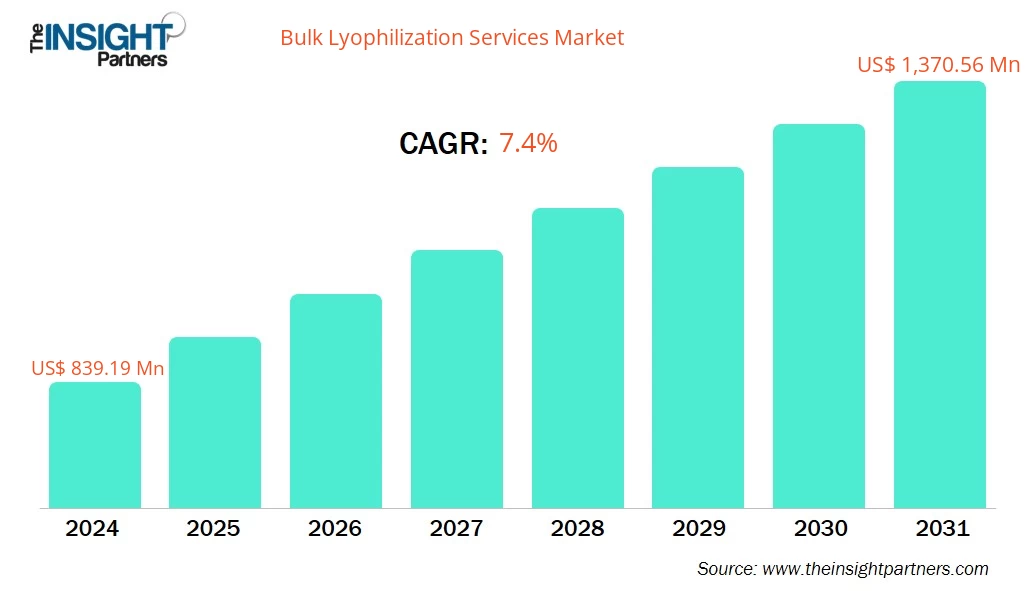
The bulk lyophilization services market size is projected to reach US$ 1,370.56 million by 2031 from US$ 839.19 million in 2024. The market is expected to register a CAGR of 7.4% during 2025–2031.
Bulk Lyophilization Services Market Analysis
The market's growth is driven by the rising demand for biologics and vaccines requiring advanced preservation, and the surging demand for outsourcing pharmaceutical manufacturing to specialized CDMOs. Technological advancements in lyophilization processes for enhanced efficiency and scalability contribute to market development. Expansion into emerging markets with improving healthcare infrastructure, adoption of lyophilization for cell and gene therapies (CGT), and integration with continuous manufacturing and process analytical technology are expected to create ample opportunities for the market in the upcoming years.
Bulk Lyophilization Services Market Overview
In the biopharmaceutical and biotech sectors, companies need to stabilize biologics that are sensitive to change—such as monoclonal antibodies, vaccines, and gene therapies— for storage and transportation that will last for a long time. Service providers are increasing their technical capabilities and capacity to meet the demand by providing batch and continuous freeze-drying cycles, which are, in most cases, supplemented by advanced automation and single-use technologies. The majority of companies choose to outsource the process of lyophilization so as not to face the high capital and regulatory burden of in-house lyophilizers. Collaborations between CDMOs and pharmaceutical developers are increasing due to the biologics pipeline and demand for customized lyophilization solutions. Enhancement of the process through AI-enabled monitoring and real-time analytics makes the process more efficient and decreases the cycle time.
Customizee This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONBulk Lyophilization Services Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Bulk Lyophilization Services Market Drivers and Opportunities
Market Drivers:
- Rising Demand for Biologics and Vaccines Requiring Advanced Preservation: The demand for bulk lyophilization to extend the stability and shelf life of biologics and vaccines has resulted from their increased production. These products, which are highly sensitive in their liquid form, are mostly converted by freeze-drying to less cold-chain-dependent powders.
- Growing Demand for Outsourcing Pharmaceutical Manufacturing to Specialized CDMOs: Bulk lyophilization of a pharmaceutical company is a task that the company usually delegates to a CDMO, thereby reaping the benefits of lowered costs and access to the right biologics solutions for its products. The operational flexibility that comes with outsourcing allows for a shorter timeline and be able to cater to the rising volumes of biologics without having to set up in-house infrastructure.
- Technological Advancements in Lyophilization Processes for Enhanced Efficiency and Scalability: Improvements such as controlled ice nucleation, QbD, microwave-assisted drying, and PAT tools contribute to the efficiency of the freeze-drying process and the quality of the product. Such changes make it possible to reduce the time taken in each cycle, at the same time ensuring uniform drying on a large scale and product stability improvement for biologics and vaccines that are complicated in nature.
Market Opportunities:
- Expansion into Emerging Markets with Improving Healthcare Infrastructure: The biopharma capacity in emerging markets is fast-growing to meet the demand for lyophilized vaccines and biologics suitable for the hot and humid environment. The investments by the government, regulatory alignment, and CDMO partnerships facilitate regional production, technology transfer, and cost-effective thermostable formulations.
- Adoption of Lyophilization for Cell and Gene Therapies (CGT): Innovations in the preservation of viral vectors and cell therapies open up opportunities for lyophilization in CGT. Enhanced stability, lowered logistics costs, and an increasing number of clinical trials fuel the demand for the special small-volume bulk freeze-dryers.
- Integration with Continuous Manufacturing and Process Analytical Technology (PAT): The collaboration of lyophilization with continuous manufacturing and PAT leads to the enhancement of the speed, consistency, and efficiency of the process. Through the support of regulatory, cycle-time reductions, automation, and flexibility in CDMOs' operations, it is possible to bring high-throughput, sustainable freeze-drying solutions for extended therapeutics to the market.
Bulk Lyophilization Services Market Report Segmentation Analysis
The bulk lyophilization services market is divided into different segments to give a clearer view of how it works, its growth potential, and the latest trends. Below is the standard segmentation approach used in industry reports:
By Product Type:
- Bulk Tray Lyophilization: Bulk tray lyophilization drives biopharma growth by ensuring stability, scalability, and efficiency for biologics, vaccines, and APIs. This progress is supported by FDA approvals, automation, sustainability, and expanding CDMO outsourcing amid rising investments in research and development.
- Drum Lyophilization: Drum lyophilization supports pharma growth through continuous drying of APIs and biologics, offering energy savings, scalability, and FDA-compliant precision amid rising generics production and increased outsourcing to CDMOs.
- Shelf Freeze-Drying: Shelf freeze-drying supports the stability, scalability, and regulatory compliance of biologics and vaccines. Its demand is fueled by advances in oncology, rising CDMO demand, adoption of QbD frameworks, and innovations that improve efficiency, sustainability, and enable decentralized manufacturing.
- Tunnel or Conveyer-Based Freeze Drying: Tunnel freeze-drying drives high-volume pharma and nutraceutical production, offering continuous, automated, energy-efficient drying with IoT monitoring, modular adaptability, regulatory compliance, and CDMO access, supporting scalability, sustainability, and global supply chains.
- Hybrid Methods: Hybrid freeze-drying combines traditional and advanced techniques to enhance speed, uniformity, and energy efficiency, supporting complex biologics, mRNA therapeutics, gene therapies, and sustainable, cost-effective clinical-scale production.
By Scale:
- Small Scale or Lab Scale
- Pilot Scale
- Commercial and Industrial Scale
By Service Type:
- Custom Process Development and Optimization
- Pilot Scale and Research and Development Lyophilization
- Full-Scale Commercial Bulk Lyophilization
By End User:
- Pharmaceutical and Biotechnology Companies
- Research and Academic Institutes
- Others
Each end user in the bulk lyophilization services market has distinct handling, safety, and regulatory needs, shaping equipment selection and operational protocols.
By Geography:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Bulk Lyophilization Services Market Regional Insights
The regional trends and factors influencing the Bulk Lyophilization Services Market throughout the forecast period have been thoroughly explained by the analysts at The Insight Partners. This section also discusses Bulk Lyophilization Services Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
Bulk Lyophilization Services Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$ 839.19 Million |
| Market Size by 2031 | US$ 1,370.56 Million |
| Global CAGR (2025 - 2031) | 7.4% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Lyophilization Format
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
Bulk Lyophilization Services Market Players Density: Understanding Its Impact on Business Dynamics
The Bulk Lyophilization Services Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
- Get the Bulk Lyophilization Services Market top key players overview
Bulk Lyophilization Services Market Share Analysis by Geography
The bulk lyophilization services market in Asia Pacific is witnessing the fastest growth, driven by increasing manufacturing capacity and investment in the biotech industry. Emerging markets in Latin America, the Middle East, and Africa have many untapped opportunities for bulk lyophilization services providers to expand.
The bulk lyophilization services market grows differently in each region owing to the economic growth and increasing demand for biologics and vaccines, followed by the surging demand for CDMOs. Below is a summary of market share and trends by region:
1. North America
- Market Share: Holds a significant portion of the global market
- Key Drivers: The biopharmaceutical R&D activities, the high production of biologics and CGT, the presence of a large number of CDMOs, strict quality standards set by the FDA, and the increasing demand for stable and temperature-resistant formulations that are compatible with decentralized distribution.
- Trends: Increasing consolidation and capacity expansion by CDMOs to meet high biologics and vaccine demand.
2. Europe
- Market Share: Substantial share due to early adoption of Bulk Lyophilization Services
- Key Drivers: The efficient vaccine manufacturing, strict regulatory frameworks of the EMA, early adoption of advanced freeze-drying technology, growing network of CDMOs, and the increase in demand for lyophilized biologics which are in line with the sustainability and continuous manufacturing initiatives.
- Trends: Emphasis on regulatory compliance and quality, driving investment in GMP lyophilization capacity
3. Asia Pacific
- Market Share: Fastest-growing region with rising market share every year
- Key Drivers: The development of biopharma infrastructure, government incentives, the rise in vaccine production, cost-effective manufacturing, and the strong use of lyophilization in the production of heat-sensitive biologics in tropical regions.
- Trends: Favorable government policies, incentives, and industrial parks supporting biopharma and lyophilization infrastructure
4. South and Central America
- Market Share: Growing market with steady progress
- Key Drivers: The increasing government support for domestic vaccine production, the expansion of public immunization programs, cold-chain challenges, and CDMO collaborations that facilitate the cost-efficient lyophilization of biologics and biosimilars.
- Trends: Partnerships between local CDMOs and global players to scale lyophilization for biologics and vaccines
5. Middle East and Africa
- Market Share: Although small, but growing quickly
- Key Drivers: The increasing public-health funding, regional vaccine initiatives, the rise of cold-chain issues, and the establishment of partnerships with global CDMOs to facilitate local capacity-building for the production of thermostable biologics, which are suitable for harsh climates.
- Trends: Investment by local governments and international organizations to build lyophilization capacity.
Bulk Lyophilization Services Market Players Density: Understanding Its Impact on Business Dynamics
High Market Density and Competition
Competition is strong due to the presence of established players, such as Hudson Valley Lyomac, Alcami Corporation, Affinity Life Sciences, OFD Life Sciences, and Quality BioResources. Regional and niche also add to the competitive landscape across different regions.
This high level of competition urges companies to stand out by offering:
- Advanced security features
- Value-added services such as Analytics & predictive maintenance, real‑time operational analytics, and installation
- Competitive pricing models
- Strong customer support and easy integration
Opportunities and Strategic Moves
- Biologic drugs in the pharmaceutical pipeline are growing rapidly, and this includes monoclonal antibodies, vaccines, and gene therapies. This growth results in an increasing demand for bulk lyophilization since these products have to be stabilized and their shelf life has to be extended. There is a rising preference for lyophilized injectables due to enhanced stability and easier reconstitution. The research applications are also expanding, thus necessitating the use of lyophilization processes.
- Companies in the biopharmaceutical industry are choosing to outsource the production of their products to specialized CMOs to reduce their capital expenditure and focus on their core competencies. The increase in biopharmaceutical production and exports drives the growth of pharmaceutical producing countries, i.e., China and India. An environmentally friendly, energy-efficient lyophilization technology is emphasized for sustainability.
- An advanced monitoring system is used to keep track of the temperature and pressure in real-time, which is very helpful in product quality and cycle optimization. Automation and robotics are used for contamination-free loading/unloading and reduced operational costs.
Other companies analyzed during the course of research:
- Biopharma Group
- Meribel Pharma
- Affinity Life Sciences
- Catalent Pharma Solutions
- Aenova Group
- PYRAMID Laboratories, Inc.
- Fresenius Kabi Contract Manufacturing
- Pfizer CentreOne
- Eurofins CDMO
- Oncomed Manufacturing A.S
- Dalton Pharma Services
- GILYOS (lyophilization specialist)
- Jubilant HollisterStier
- Biopharma Process Systems
- Piramal Pharma Solutions
- Biofortuna
- Comser (contract freeze-drying)
- INCOG BioPharma Services
- Rentschler Biopharma SE
- Therapure BioPharma Inc.
- Enzene
- SCTbio
- AbbVie Contract Manufacturing
- Scantibodies Laboratory, Inc.
Bulk Lyophilization Services Market News and Recent Developments
- Catachem Inc. Doubles Lyophilization Capacity with Advanced Millrock Technology: Catachem Inc., a reagent manufacturer and provider of custom lyophilization services, announced a significant expansion in its production capabilities with the installation of a customized lyophilization machine from Millrock Technology, Inc. This strategic investment marks a pivotal enhancement in Catachem’s commitment to delivering high-quality lyophilized products to the biotech industry.
- Alcami Partners with Tanvex CDMO to Offer a Complete Solution for Biologics Developers: Alcami Corporation announced a new strategic partnership with Tanvex CDMO, a biologics developer offering pre-clinical to commercial biologic CDMO services. Through the collaboration, Alcami and Tanvex can offer clients a complete solution from bulk drug substance production through to finished drug product, pairing Alcami’s expertise in liquid and lyophilization sterile filling, packaging, and labeling with Tanvex’s high-throughput bulk drug substance development and manufacturing services.
Bulk Lyophilization Services Market Report Coverage and Deliverables
The "Bulk Lyophilization Services Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Bulk Lyophilization Services Market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Bulk Lyophilization Services Market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Bulk Lyophilization Services Market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the Bulk Lyophilization Services Market
- Detailed company profiles
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Recent Reports
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends




















 Get Free Sample For
Get Free Sample For