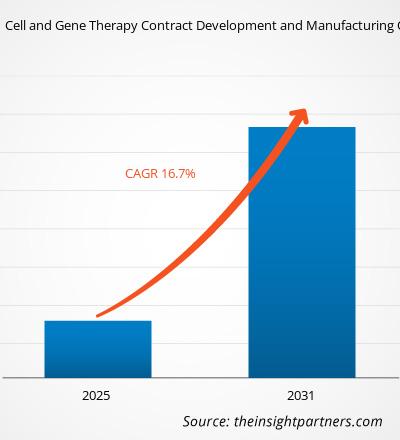
The cell and gene therapy contract development and manufacturing organization market size is projected to reach US$ 31.86 billion by 2031 from US$ 6.22 billion in 2024. The market is expected to register a CAGR of 26.4% during 2025–2031. The rising integration of AI and digital transformation is likely to bring in new market trends during the forecast period.
Cell and Gene Therapy Contract Development and Manufacturing Organization Market Analysis
Cell and gene therapies require specialized manufacturing processes, including the production of viral vectors, transduced cells, and other specialized biological materials. These therapies target rare or complex diseases, such as genetic disorders, cancer, and autoimmune diseases. According to the Alliance for Regenerative Medicine (ARM), the total number of clinical trials focused on gene therapies alone reached over 1,000 as of 2024, with hundreds more in the pipeline, creating the demand for contract development and manufacturing organizations (CDMOs).
The increasing number of biotech startups and biopharmaceutical companies entering the gene and cell therapy field has propelled the demand for CDMOs. Biopharmaceutical companies, especially small and medium-sized enterprises, lack the infrastructure and expertise required for manufacturing these specialized therapies. Consequently, they turn to CDMOs for their comprehensive expertise in managing clinical trial production, ensuring regulatory compliance, and scaling up manufacturing processes. The Autologus Therapeutics and AGC Biologics Milan Partnership started in 2020 when the company was involved in the development, manufacturing, and supply of viral vectors for Autolus’s obe-cel CAR-T product candidate, AUCATZYL. The collaboration between the two parties was instrumental in bringing the therapy to market in a timely manner. In May 2025, Astraveus SAS entered into a strategic partnership with the Netherlands Center for the Clinical Advancement of Stem Cell and Gene Therapies (NecstGen) to evaluate the Lakhesys Benchtop Cell Factory for the manufacturing of CAR-T therapies.
Technological advancements such as artificial intelligence (AI) optimize manufacturing processes for clinical trials. These innovations enable efficient and cost-effective production methods, which are critical based on the complexities involved. Thus, increasing clinical trials for innovative therapies, along with increasing demand for CDMOs for increasing research, development, and commercialization, drive the growth of the cell and gene therapy contract development and manufacturing organization market.
Cell and Gene Therapy Contract Development and Manufacturing Organization Market Overview
The cell and gene therapy contract development and manufacturing organization market is expanding due to the increasing clinical trials for innovative therapies and surging regulatory approvals and commercialization. Prominent players operating in the market are focusing on innovations and collaborative efforts for enhanced product availability and reach. In April 2025, AGC Biologics Launched a New Dedicated Cell and Gene Business Division. The new Cell and Gene Technologies Division will focus on elevating existing AGC Biologics capabilities and supporting developers who require capacity, scientific capabilities, and technically qualified cell and gene CDMO operators. The AGC Biologics Milan Cell and Gene Center of Excellence will be the central location for this new Division. The site offers 30 years of experience in cell and gene therapy, with nine commercial approvals and hundreds of GMP batches produced successfully. However, the high manufacturing complexities in cell and gene therapy contract development and manufacturing organizations hinder market growth.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Cell and Gene Therapy Contract Development and Manufacturing Organization Market: Strategic Insights
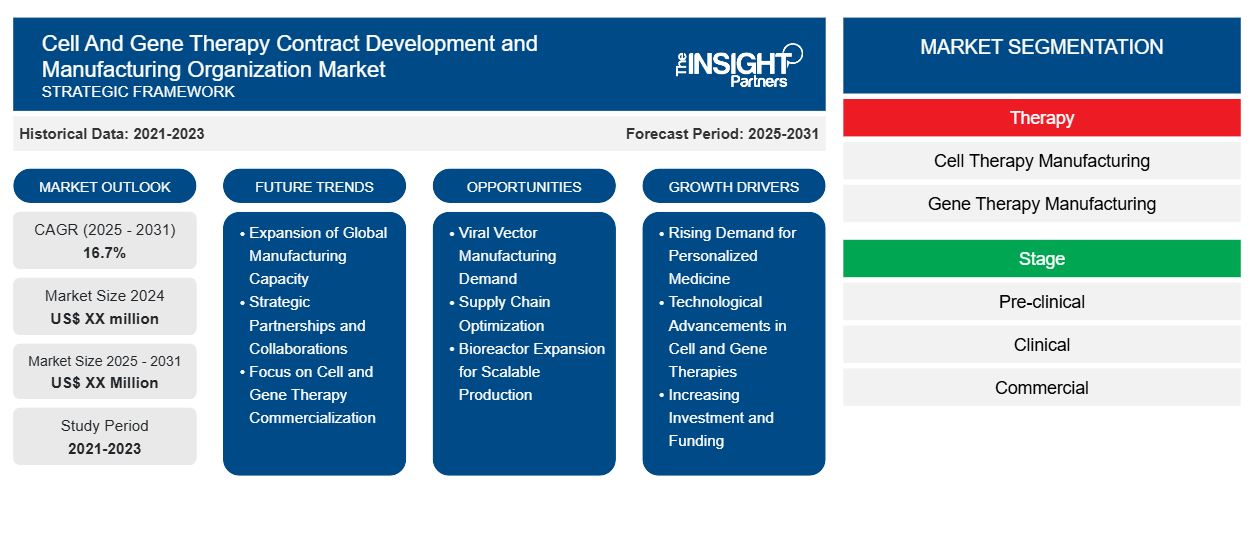
- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Customize This Report To Suit Your Requirement
You will get customization on any report - free of charge - including parts of this report, or country-level analysis, Excel Data pack, as well as avail great offers and discounts for start-ups & universities
Cell and Gene Therapy Contract Development and Manufacturing Organization Market: Strategic Insights

- Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
Cell and Gene Therapy Contract Development and Manufacturing Organization Market Drivers and Opportunities
Surging Regulatory Approvals and Commercialization
Over the past few years, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have streamlined their approval processes for cell and gene therapies. The expedited process enables faster market entry for innovative therapies and increases the demand for specialized manufacturing services provided by CDMOs. One of the primary reasons for this change is the recognition of cell and gene therapies in the treatment of diseases that were previously untreatable. The FDA has introduced the Regenerative Medicine Advanced Therapy (RMAT) designation and the Breakthrough Therapy Designation, which expedite the development and review of promising therapies. These designations are helpful in the faster approval of cell and gene therapies. Zolgensma, a gene therapy for spinal muscular atrophy (SMA), received its first FDA approval in 2019. By 2024, it will be approved in 51 other countries in just four years, a process that would typically take much longer. This rapid approval represents the regulatory push to bring life-changing therapies to market. According to the Alliance for Regenerative Medicine (ARM), over 24 gene therapies have received regulatory approval globally since 2020, with many more in the pipeline.
Access to Specialized Facilities and Technologies
The demand for cell and gene therapies is driving biopharmaceutical companies toward CDMOs that can provide cutting-edge facilities and technologies, which are required to scale up production while ensuring quality, compliance, and regulatory approval. These specialized capabilities are essential for the production of complex and personalized therapies, which require advanced infrastructure to maintain high manufacturing standards. Cell and gene therapies, including gene editing, viral vector production, and personalized medicine, require specialized facilities equipped with the latest technologies. The production of viral vectors used in gene therapies requires GMP standard facilities to ensure the safety, consistency, and quality of the final product. These facilities should be equipped to handle live cells and genetically modified organisms in controlled and monitored environments. The increasing adoption of automated cell culture systems, continuous manufacturing, and digital quality monitoring systems enhances the growth and efficiency of gene therapy production. In March 2025, Bharat Biotech invested US$ 75 million in its first cell and gene therapy facility in southern India. It is expected to launch new therapies in the next 3 years for oncology and rare diseases.
The need for specialized facilities creates a substantial opportunity for CDMOs. It is cost-effective and efficient for biopharma companies to collaborate with CDMOs that have the required technology and facilities. In 2023, Bristol-Myers Squibb collaborated with a CDMO for the production of its CAR-T cell therapy, Breyanzi. This therapy involves harvesting, modifying, and expanding a patient’s T-cells, a process requiring specialized technology and facilities to ensure the desired therapeutic effect. By leveraging CDMO expertise in specialized facilities, Bristol-Myers Squibb was able to scale production while ensuring regulatory compliance and quality. Thus, the soaring need for advanced manufacturing capabilities with advanced technology is expected to create future growth opportunities for the cell and gene therapy contract research and development organization market.
Cell and Gene Therapy Contract Development and Manufacturing Organization Market Report Segmentation Analysis
Key segments that contributed to the derivation of the cell and gene therapy contract development and manufacturing organization market analysis are service type, product type, and end user.
- Based on service type, the cell and gene therapy contract development and manufacturing organization market is segmented into drug development and manufacturing, testing and regulatory services, and others. The drug development and manufacturing segment held the largest share of the market in 2024.
- In terms of product type, the cell and gene therapy contract development and manufacturing organization market is bifurcated into gene therapy and cell therapy. The cell therapy segment dominated the market in 2024.
- By end user, the cell and gene therapy contract development and manufacturing organization market is categorized into pharmaceutical companies, biopharmaceutical companies, and others. The biopharmaceutical companies segment dominated the market in 2024.
Cell and Gene Therapy Contract Development and Manufacturing Organization Market Share Analysis by Geography
The geographic scope of the cell and gene therapy contract development and manufacturing organization market report mainly focuses on five regions: North America, Asia Pacific, Europe, South & Central America, and the Middle East & Africa. In terms of revenue, North America dominated the market in 2024. It is expected to continue its dominance in the global market during the forecast period. The US is observing growing advancements in biotechnology, an increasing prevalence of genetic diseases, and surging demand for specialized manufacturing services. As per the estimates of the US Government Accountability Office published in October 2021, ~25 to 30 million people suffer from rare diseases in the country. As per the Food and Drug Administration (FDA), more than 7,000 rare diseases affect over 30 million people in the country. The growing understanding of these diseases has led to a surge in gene therapy development. CDMOs play a crucial role in this space by providing specialized services for the development and manufacturing of gene therapies targeting rare genetic disorders.
In 2023, the US FDA approved numerous cell and gene therapies, including gene-editing treatments targeting rare diseases. Therapies such as exagamglogene autotemcel (Casgevy) and lovotibeglogene autotemcel for sickle cell disease, as well as valoctocogene roxaparvovec for severe hemophilia A, have received FDA approval, highlighting the potential of gene therapies in addressing rare disease challenges. The accelerated regulatory pathways, such as the Regenerative Medicine Advanced Therapy (RMAT) designation, have spurred biotechnology companies to partner with CDMOs for scaling production.
Investments in manufacturing infrastructure have bolstered the market growth. The National Cell Manufacturing Consortium, established through collaboration between 15 academic institutes, over 25 companies, and government agencies, aims to enable cost-effective, large-scale manufacturing of cell therapies. In addition, strategic collaborations between CDMOs, academic institutions, and biopharma companies also contribute to market growth.
Cell and Gene Therapy Contract Development and Manufacturing Organization Market Regional Insights
The regional trends and factors influencing the Cell and Gene Therapy Contract Development and Manufacturing Organization Market throughout the forecast period have been thoroughly explained by the analysts at Insight Partners. This section also discusses Cell and Gene Therapy Contract Development and Manufacturing Organization Market segments and geography across North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America.
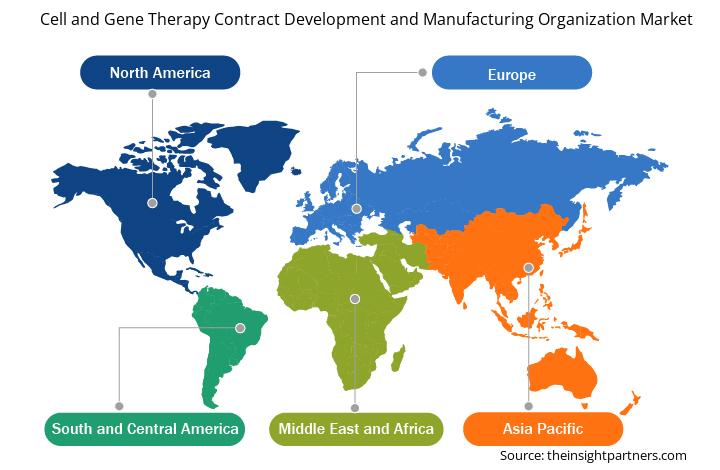
- Get the Regional Specific Data for Cell and Gene Therapy Contract Development and Manufacturing Organization Market
Cell and Gene Therapy Contract Development and Manufacturing Organization Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2024 | US$�6.22 Billion |
| Market Size by 2031 | US$�31.86 Billion |
| Global CAGR (2025 - 2031) | 26.4% |
| Historical Data | 2021-2023 |
| Forecast period | 2025-2031 |
| Segments Covered |
By Service Type
|
| Regions and Countries Covered | North America
|
| Market leaders and key company profiles |
Cell and Gene Therapy Contract Development and Manufacturing Organization Market Players Density: Understanding Its Impact on Business Dynamics
The Cell and Gene Therapy Contract Development and Manufacturing Organization Market market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Market players density refers to the distribution of firms or companies operating within a particular market or industry. It indicates how many competitors (market players) are present in a given market space relative to its size or total market value.
Major Companies operating in the Cell and Gene Therapy Contract Development and Manufacturing Organization Market are:
- WuXi Biologics Inc
- Charles River Laboratories International Inc
- Catalent Inc
- Lonza Group AG
- Thermo Fisher Scientific Inc.
- AGC Biologics AS
Disclaimer: The companies listed above are not ranked in any particular order.
- Get the Cell and Gene Therapy Contract Development and Manufacturing Organization Market top key players overview
Cell and Gene Therapy Contract Development and Manufacturing Organization Market News and Recent Developments
The cell and gene therapy contract development and manufacturing organization market is evaluated by gathering qualitative and quantitative data post primary and secondary research, which includes important corporate publications, association data, and databases. Below are key developments witnessed in the cell and gene therapy contract development and manufacturing organization market:
- WuXi Biologics Launches EffiX Microbial Expression Platform to Boost Recombinant Protein and Plasmid DNA Production. (Source: WuXi Biologics, March 2025)
- Charles River Laboratories International, Inc. and AAVantgarde announced a CDMO agreement to produce Good Manufacturing Practice (GMP) plasmid DNA. AAVantgarde, a clinical-stage biotechnology company with two proprietary adeno-associated viral (AAV) vector platforms for large gene delivery and developing products to treat inherited retinal diseases, will leverage Charles River’s expertise in manufacturing GMP plasmid DNA. (Source: Charles River Laboratories International, Inc., July 2024)
- Siren Biotechnology, pioneer of Universal AAV Immuno-Gene Therapy for Cancer, entered into a strategic partnership with Catalent Inc., the leader in enabling the development and supply of better treatments for patients worldwide, to support the development and manufacturing of Siren Biotechnology’s AAV immuno-gene therapies. (Source: Catalent Inc., Press Release, May 2024)
Cell and Gene Therapy Contract Development and Manufacturing Organization Market Report Coverage and Deliverables
The "Cell and Gene Therapy Contract Development and Manufacturing Organization Market Size and Forecast (2021–2031)" report provides a detailed analysis of the market covering below areas:
- Wound closure devices market size and forecast at global, regional, and country levels for all the key market segments covered under the scope
- Wound closure devices market trends, as well as market dynamics such as drivers, restraints, and key opportunities
- Detailed PEST and SWOT analysis
- Wound closure devices market analysis covering key market trends, global and regional framework, major players, regulations, and recent market developments
- Industry landscape and competition analysis covering market concentration, heat map analysis, prominent players, and recent developments for the cell and gene therapy contract development and manufacturing organization market
- Detailed company profiles
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset


- Artificial Intelligence in Defense Market
- Ceramic Injection Molding Market
- Mesotherapy Market
- Artwork Management Software Market
- Smart Grid Sensors Market
- Greens Powder Market
- Wheat Protein Market
- Social Employee Recognition System Market
- Electronic Health Record Market
- Electronic Toll Collection System Market

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
This text is related
to segments covered.

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
This text is related
to country scope.
Frequently Asked Questions
North America dominated the market in 2024.
Increasing clinical trials for innovative therapies and surging regulatory approvals and commercialization are significant factors fueling the market growth.
The rising integration of AI and digital transformation is likely to emerge as a new trend in the market in the coming years.
The market is expected to register a CAGR of 26.4% during 2025–2031.
The market value is expected to reach US$ 31.86 billion by 2031.
WuXi Biologics Inc, Charles River Laboratories International Inc, Catalent Inc, Lonza Group AG, Thermo Fisher Scientific Inc., AGC Biologics AS, Takara Bio Inc, FUJIFILM Holdings Corp, Pluri Inc, SK pharmteco Inc, Aenova Holding GmbH, and Minaris Advanced Therapies are key players operating in the market.
Trends and growth analysis reports related to Life Sciences : READ MORE..
The List of Companies - Cell and Gene Therapy Contract Development and Manufacturing Organization Market
- WuXi Biologics Inc
- Charles River Laboratories International Inc
- Catalent Inc
- Lonza Group AG
- Thermo Fisher Scientific Inc.
- AGC Biologics AS
- Takara Bio Inc
- FUJIFILM Holdings Corp
- Pluri Inc
- SK pharmteco Inc
- Aenova Holding GmbH
- Minaris Advanced Therapies
 Get Free Sample For
Get Free Sample For