CIN & HR-HPV Treatment Market Size and Competitive Analysis by 2028
CIN & HR-HPV Treatment Market Forecast to 2028 - Industry Analysis By Disease Type (Cervical Intraepithelial Neoplasia 1, Cervical Intraepithelial Neoplasia 2, and Cervical Intraepithelial Neoplasia 3), Strain Type (HPV 16, HPV 18, and Others), Offering (Diagnostic Method and Treatment), Product Type (Kits & Reagents, Instruments, and Services), and End User (Hospitals & Clinics, Diagnostic Laboratories, Specialized Clinical Laboratories, and Others)
Historic Data: 2020-2021 | Base Year: 2022 | Forecast Period: 2023-2028- Report Date : May 2023
- Report Code : TIPRE00029878
- Category : Life Sciences
- Status : Published
- Available Report Formats :


- No. of Pages : 245
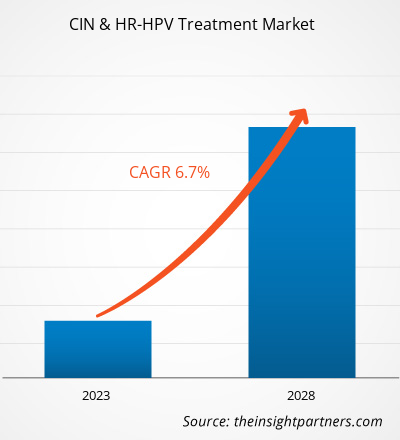
[Research Report] The CIN & HR-HPV treatment market size is expected to reach US$ 17,164.61 million by 2028 from US$ 11,635.17 million in 2022; the market is estimated to record a CAGR of 6.7% from 2023 to 2028.
The CIN & HR-HPV treatment market is segmented on the basis of disease type, strain type, offerings, product type, end user, and geography. The report offers insights and in-depth analysis of the market, emphasizing parameters such as drivers, trends, and opportunities in the market and competitive landscape analysis of leading market players across various regions. It also includes analyses of the impact of the COVID-19 pandemic across major regions.
Market Insights
Favorable Initiatives for Preventing Cervical Cancer Drives CIN & HR-HPV Treatment Market Growth
In August 2020, the World Health Organization (WHO) devised a global strategy for the elimination of cervical cancer. Per this strategy, all countries in the world must focus on reaching and maintaining an incidence rate of less than 4 per 100,000 women to eliminate cervical cancer. To attain such low incidence rates, countries should focus on cervical cancer screening, treatment, and vaccination. Medicare, a popular government insurance program, provides coverage for PAP tests, pelvic exams, and clinical breast examinations performed every two years for cervical cancer screening.
Unitaid, a global health agency that works to bring about innovative solutions to prevent, diagnose, and treat major diseases in low- and middle-income countries, has come up with a new initiative for cervical cancer prevention. Its efforts are focused on expanding access to critical tools and services to screen for the early signs of cervical cancer, followed by the treatment of positive cases. Unitaid is providing support for the Clinton Health Access Initiative (CHAI) and the Scale Up Cervical Cancer Elimination with Secondary Prevention Strategy (SUCCESS) Project, which are being carried out in partnership with Expertise France, Jhpiego, and the Union for International Cancer Control (UICC). Unitaid is collaborating with these partners and governments of 14 low- and middle-income countries to develop an affordable and highly effective package of tools, which can help the World Health Organization achieve its cervical cancer elimination targets. The Unitaid has invested ~US$ 70 million in innovative tools to screen women living in low-resource environments for precancer conditions and treat them. In 14 countries, Unitaid is striving to overcome access barriers and laying the groundwork for national cervical cancer elimination efforts, demonstrating effective models of prevention across low- and middle-income countries.
Customize This Report To Suit Your Requirement
Get FREE CUSTOMIZATIONCIN & HR-HPV Treatment Market: Strategic Insights
-
Get Top Key Market Trends of this report.This FREE sample will include data analysis, ranging from market trends to estimates and forecasts.
In India, screening methods such as cytology, co-testing (HPV + cytology), primary HPV testing, and visual inspection with acetic acid are employed in various settings, according to resource availability and compliance standards. The Federation of Obstetricians and Gynaecologists of India (FOGSI) has published good clinical practice recommendations (GCPR) for the management of screen-positive women in different resource settings. RMNCH + A (which stands for Reproductive Maternal Neonatal Childhood Health + Adolescent Health + Adolescent) is the strategy of the National Health Mission of India (NHM) that encourages small family norms. This strategy involves delivering contraceptives at home by Accredited Social Health Activists (ASHA), delaying marriage age, promoting menstrual hygiene and sexual hygiene awareness, and prompting the treatment of reproductive tract illnesses in "Suraksha Clinics."
Such initiatives by various healthcare organizations and governments for preventing cervical cancer boost the growth of the CIN & HR-HPV treatment market.
Disease Type-Based Insights
Based on disease type, the CIN & HR-HPV treatment market is segmented into cervical intraepithelial neoplasia 1 (CIN 1), cervical intraepithelial neoplasia 2 (CIN 2), and cervical intraepithelial neoplasia 3 (CIN 3). In 2022, the cervical intraepithelial neoplasia 3 (CIN 3) segment held the largest market share, and it is anticipated to register the highest CAGR during the forecast period.
Strain Type-Based Insights
Based on strain type, the CIN & HR-HPV treatment market is segmented into HPV 16, HPV 18, and others. The HPV 16 segment held the largest share of the market in 2022. Th HPV 18 segment is expected to record the highest CAGR during the forecast period.
Offering-Based Insights
Based on offering, the CIN & HR-HPV treatment market is categorized into diagnostic method and treatment. In 2022, the treatment segment held a larger share of the market. However, diagnostic method segment is expected to register a higher CAGR during the forecast period. Further, the diagnostic method segment is further categorized into HPV testing, Pap smear test, colposcopy, and biopsy. The HPV testing segment held the largest share on the market for diagnostic method in 2022. In addition, the treatment segment is bifurcated into excisional surgery and ablative techniques. In 2022, the ablative techniques segment held a larger share of the market and is anticipated to register a higher CAGR in the market during the forecast period.
Product Type-Based Insights
Based on product type, the CIN & HR-HPV treatment market is segmented into kits & reagents, instruments, and services. The services segment held the largest share of the market in 2022, and it is expected to record the highest CAGR during the forecast period.
End User-Based Insights
Based on end user, the CIN & HR-HPV treatment market is segmented into hospitals & clinics, diagnostic laboratories, specialized clinical laboratories, and others. The hospital & clinics segment accounted for the largest market share in 2022. The diagnostic laboratories segment is expected to record the highest CAGR during the forecast period.
The CIN & HR-HPV treatment market players adopt organic strategies such as product launch and expansion to expand their geographic footprint and product portfolios. Additionally, these growth strategies help companies strengthen their clientele and enlarge their product portfolios.
- In October 2022, Fujirebio Europe and Self-screen BV formed a commercial collaboration to distribute the PreCursor-M+ methylation-specific molecular in-vitro diagnosis (IVD) assay (a trademark of Self-screen BV). The test is intended for qualitatively detecting the elevated methylation levels of cervical cancer biomarkers. It may be used as a triage follow-up test on women with HPV-positive results and ASCUS/LSIL cytology. The PreCursor-M+ assay completes Fujirebio's HPV-specific molecular test portfolio.
- In April 2021, Roche received US FDA approval for the fully automated, high throughput cobas 6800/8800 Systems for their use in HPV testing. The cobas HPV test identifies women at risk of cervical cancer by detecting the presence of hrHPV DNA in cervical samples. cobas HPV is indicated for routine cervical cancer screening per professional medical guidelines, including triage of ASC-US cytology and primary HPV screening of women to assess the risk for cervical precancer and cancer.
CIN and HR-HPV Treatment
CIN & HR-HPV Treatment Market Report Scope
| Report Attribute | Details |
|---|---|
| Market size in 2022 | US$ 11.64 Billion |
| Market Size by 2028 | US$ 17.16 Billion |
| Global CAGR (2022 - 2028) | 6.7% |
| Historical Data | 2020-2021 |
| Forecast period | 2023-2028 |
| Segments Covered |
By Disease Type
|
| Regions and Countries Covered |
North America
|
| Market leaders and key company profiles |
|
CIN & HR-HPV Treatment Market Players Density: Understanding Its Impact on Business Dynamics
The CIN & HR-HPV Treatment Market is growing rapidly, driven by increasing end-user demand due to factors such as evolving consumer preferences, technological advancements, and greater awareness of the product's benefits. As demand rises, businesses are expanding their offerings, innovating to meet consumer needs, and capitalizing on emerging trends, which further fuels market growth.
Company Profiles
- Fujirebio Europe NV
- Qiagen NV
- Zilico Ltd
- Abbott Laboratories
- Cepheid
- F. Hoffmann-La Roche Ltd
- INOVIO Pharmaceuticals Inc
- Bioneer Corp
- Antiva Biosciences Inc
- Thermo Fisher Scientific Inc.
Frequently Asked Questions
Mrinal is a seasoned research analyst with over 8 years of experience in Life Sciences Market Intelligence and Consulting. With a strategic mindset and unwavering commitment to excellence, she has built deep expertise in pharmaceutical forecasting, market opportunity assessment, and developing industry benchmarks. Her work is anchored in delivering actionable insights that empower clients to make informed strategic decisions.
Mrinal’s core strength lies in translating complex quantitative datasets into meaningful business intelligence. Her analytical acumen is instrumental in shaping go-to-market (GTM) strategies and uncovering growth opportunities across the pharmaceutical and medical device sectors. As a trusted consultant, she consistently focuses on streamlining workflow processes and establishing best practices, thereby driving innovation and operational efficiency for her clients.
- Historical Analysis (2 Years), Base Year, Forecast (7 Years) with CAGR
- PEST and SWOT Analysis
- Market Size Value / Volume - Global, Regional, Country
- Industry and Competitive Landscape
- Excel Dataset
Testimonials
The Insight Partners' SCADA System Market report is comprehensive, with valuable insights on current trends and future forecasts. The team was highly professional, responsive, and supportive throughout. We are very satisfied and highly recommend their services.
RAN KEDEM Partner, Reali Technologies LTDsI requested a report on a very specific software market and the team produced the report in a few days. The information was very relevant and well presented. I then requested some changes and additions to the report. The team was again very responsive and I got the final report in less than a week.
JEAN-HERVE JENN Chairman, Future AnalyticaWe worked with The Insight Partners for an important market study and forecast. They gave us clear insights into opportunities and risks, which helped shape our plans. Their research was easy to use and based on solid data. It helped us make smart, confident decisions. We highly recommend them.
PIYUSH NAGPAL Sr. Vice President, High Beam GlobalThe Insight Partners delivered insightful, well-structured market research with strong domain expertise. Their team was professional and responsive throughout. The user-friendly website made accessing industry reports seamless. We highly recommend them for reliable, high-quality research services
YUKIHIKO ADACHI CEO, Deep Blue, LLC.This is the first time I have purchased a market report from The Insight Partners.While I was unsure at first, I visited their web site and felt more comfortable to take the risk and purchase a market report.I am completely satisfied with the quality of the report and customer service. I had several questions and comments with the initial report, but after a couple of dialogs over email with their analyst I believe I have a report that I can use as input to our strategic planning process.Thank you so much for taking the extra time and making this a positive experience.I will definitely recommend your service to others and you will be my first call when we need further market data.
JOHN SUZUKI President and Chief Executive Officer, Board Director, BK TechnologiesI wish to appreciate your support and the professionalism you displayed in the course of attending to my request for information regarding to infectious disease IVD market in Nigeria. I appreciate your patience, your guidance, and the fact that you were willing to offer a discount, which eventually made it possible for us to close a deal. I look forward to engaging The Insight Partners in the future, all thanks to the impression you have created in me as a result of this first encounter.
DR CHIJIOKE ONYIA MANAGING DIRECTOR, PineCrest Healthcare Ltd.Reason to Buy
- Informed Decision-Making
- Understanding Market Dynamics
- Competitive Analysis
- Identifying Emerging Markets
- Customer Insights
- Market Forecasts
- Risk Mitigation
- Boosting Operational Efficiency
- Strategic Planning
- Investment Justification
- Tracking Industry Innovations
- Aligning with Regulatory Trends





 Get Free Sample For
Get Free Sample For